Higher chromatin mobility supports totipotency and precedes pluripotency in vivo
- PMID: 24831699
- PMCID: PMC4035533
- DOI: 10.1101/gad.238881.114
Higher chromatin mobility supports totipotency and precedes pluripotency in vivo
Abstract
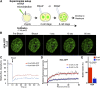
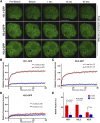
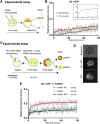
The fusion of the gametes upon fertilization results in the formation of a totipotent cell. Embryonic chromatin is expected to be able to support a large degree of plasticity. However, whether this plasticity relies on a particular conformation of the embryonic chromatin is unknown. Moreover, whether chromatin plasticity is functionally linked to cellular potency has not been addressed. Here, we adapted fluorescence recovery after photobleaching (FRAP) in the developing mouse embryo and show that mobility of the core histones H2A, H3.1, and H3.2 is unusually high in two-cell stage embryos and decreases as development proceeds. The transition toward pluripotency is accompanied by a decrease in histone mobility, and, upon lineage allocation, pluripotent cells retain higher mobility than the differentiated trophectoderm. Importantly, totipotent two-cell-like embryonic stem cells also display high core histone mobility, implying that reprogramming toward totipotency entails changes in chromatin mobility. Our data suggest that changes in chromatin dynamics underlie the transitions in cellular plasticity and that higher chromatin mobility is at the nuclear foundations of totipotency.
Keywords: cell fate; chromatin dynamics; pluripotency; reprogramming; totipotent cells.
© 2014 Bošković et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Burton A, Muller J, Tu S, Padilla-Longoria P, Guccione E, Torres-Padilla ME 2013. Single-cell profiling of epigenetic modifiers identifies PRDM14 as an inducer of cell fate in the mammalian embryo. Cell Rep 5: 687–701 - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A 2003. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113: 643–655 - PubMed
-
- Cremer T, Cremer C 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2: 292–301 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
