The tetraspanin CD53 modulates responses from activating NK cell receptors, promoting LFA-1 activation and dampening NK cell effector functions
- PMID: 24832104
- PMCID: PMC4022634
- DOI: 10.1371/journal.pone.0097844
The tetraspanin CD53 modulates responses from activating NK cell receptors, promoting LFA-1 activation and dampening NK cell effector functions
Abstract
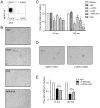
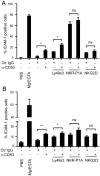
NK cells express several tetraspanin proteins, which differentially modulate NK cell activities. The tetraspanin CD53 is expressed by all resting NK cells and was previously shown to decrease NK cell cytotoxicity upon ligation. Here, we show that CD53 ligation reduced degranulation of rat NK cells in response to tumour target cells, evoked redirected inhibition of killing of Fc-bearing targets, and reduced the IFN-γ response induced by plate-bound antibodies towards several activating NK cell receptors (Ly49s3, NKR-P1A, and NKp46). CD53 induced activation of the β2 integrin LFA-1, which was further enhanced upon co-stimulation with activating NK cell receptors. Concordant with a role for CD53 in increasing NK cell adhesiveness, CD53 ligation induced a strong homotypic adhesion between NK cells. Further, the proliferative capacity of NK cells to a suboptimal dose of IL-2 was enhanced by CD53 ligation. Taken together, these data suggest that CD53 may shift NK cell responses from effector functions towards a proliferation phase.
Conflict of interest statement
Figures



Similar articles
-
IFN-gamma production and degranulation are differentially regulated in response to stimulation in murine natural killer cells.Scand J Immunol. 2008 Jan;67(1):1-11. doi: 10.1111/j.1365-3083.2007.02026.x. Epub 2007 Nov 19. Scand J Immunol. 2008. PMID: 18028287
-
Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions.Front Immunol. 2020 Feb 20;11:202. doi: 10.3389/fimmu.2020.00202. eCollection 2020. Front Immunol. 2020. PMID: 32153568 Free PMC article.
-
Frontline Science: A hyporesponsive subset of rat NK cells negative for Ly49s3 and NKR-P1B are precursors to the functionally mature NKR-P1B+ subset.J Leukoc Biol. 2017 Dec;102(6):1289-1298. doi: 10.1189/jlb.1HI0517-177RR. Epub 2017 Jul 26. J Leukoc Biol. 2017. PMID: 28747319
-
Tetraspanin CD53: an overlooked regulator of immune cell function.Med Microbiol Immunol. 2020 Aug;209(4):545-552. doi: 10.1007/s00430-020-00677-z. Epub 2020 May 21. Med Microbiol Immunol. 2020. PMID: 32440787 Free PMC article. Review.
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
Cited by
-
Tetraspanins in digestive‑system cancers: Expression, function and therapeutic potential (Review).Mol Med Rep. 2024 Nov;30(5):200. doi: 10.3892/mmr.2024.13324. Epub 2024 Sep 6. Mol Med Rep. 2024. PMID: 39239742 Free PMC article. Review.
-
Diversifying selection signatures among divergently selected subpopulations of Polish Red cattle.J Appl Genet. 2019 Feb;60(1):87-95. doi: 10.1007/s13353-019-00484-0. Epub 2019 Jan 26. J Appl Genet. 2019. PMID: 30685825 Free PMC article.
-
The versatile roles of testrapanins in cancer from intracellular signaling to cell-cell communication: cell membrane proteins without ligands.Cell Biosci. 2023 Mar 20;13(1):59. doi: 10.1186/s13578-023-00995-8. Cell Biosci. 2023. PMID: 36941633 Free PMC article. Review.
-
Antitumor Immunity Is Controlled by Tetraspanin Proteins.Front Immunol. 2018 May 29;9:1185. doi: 10.3389/fimmu.2018.01185. eCollection 2018. Front Immunol. 2018. PMID: 29896201 Free PMC article. Review.
-
A deep learning model to predict RNA-Seq expression of tumours from whole slide images.Nat Commun. 2020 Aug 3;11(1):3877. doi: 10.1038/s41467-020-17678-4. Nat Commun. 2020. PMID: 32747659 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

