The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo
- PMID: 24832194
- PMCID: PMC4023132
- DOI: 10.1038/srep04986
The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo
Abstract
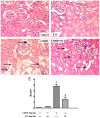
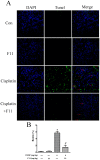
The clinical use of cisplatin was severely limited by its associated nephrotoxicity. In this study, we investigated whether the pseudoginsenoside F11 had protective effects against cisplatin-induced nephrotoxicity. To clarify it, one in vivo model of cisplatin-induced acute renal failure was performed. The results showed that pretreatment with F11 reduced cisplatin-elevated blood urea nitrogen and creatinine levels, as well as ameliorated the histophathological damage. Further studies showed that F11 could suppress P53 activation, inverse the ratio of Bax/Bcl2 and the anti-oxidative and free radical levels induced by cisplatin, which in turn inhibited tubular cell apoptosis. Importantly, F11 enhanced rather than inhibited the anti-tumor activity of cispaltin in murine melanoma and Lewis lung cancer xenograft tumor models. Our findings suggested that administering F11 with cisplatin might alleviate the associated nephrotoxicity without compromising its therapeutic efficiency. This finding provides a novel potential strategy in the clinical treatment of cancer.
Figures




References
-
- Boulikas T. & Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 10, 1663–1682 (2003). - PubMed
-
- Bose R. N. Biomolecular targets for platinum antitumor drugs. Mini Rev Med Chem 2, 103–111 (2002). - PubMed
-
- Arany I. & Safirstein R. L. Cisplatin nephrotoxicity. Semin Nephrol 23, 460–464 (2003). - PubMed
-
- Perazella M. A. & Moeckel G. W. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol 30, 570–581 (2010). - PubMed
-
- Yao X., Panichpisal K., Kurtzman N. & Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 334, 115–124 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

