T cell inactivation by poxviral B22 family proteins increases viral virulence
- PMID: 24832205
- PMCID: PMC4022744
- DOI: 10.1371/journal.ppat.1004123
T cell inactivation by poxviral B22 family proteins increases viral virulence
Abstract
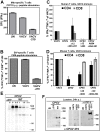
Infections with monkeypox, cowpox and weaponized variola virus remain a threat to the increasingly unvaccinated human population, but little is known about their mechanisms of virulence and immune evasion. We now demonstrate that B22 proteins, encoded by the largest genes of these viruses, render human T cells unresponsive to stimulation of the T cell receptor by MHC-dependent antigen presentation or by MHC-independent stimulation. In contrast, stimuli that bypass TCR-signaling are not inhibited. In a non-human primate model of monkeypox, virus lacking the B22R homologue (MPXVΔ197) caused only mild disease with lower viremia and cutaneous pox lesions compared to wild type MPXV which caused high viremia, morbidity and mortality. Since MPXVΔ197-infected animals displayed accelerated T cell responses and less T cell dysregulation than MPXV US2003, we conclude that B22 family proteins cause viral virulence by suppressing T cell control of viral dissemination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, et al. (2005) A tale of two clades: monkeypox viruses. J Gen Virol 86: 2661–2672. - PubMed
-
- Hutson CL, Olson VA, Carroll DS, Abel JA, Hughes CM, et al. (2009) A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol 90: 323–333. - PubMed
-
- Sbrana E, Xiao SY, Newman PC, Tesh RB (2007) Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am J Trop Med Hyg 76: 155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

