A mutation in the FAM83G gene in dogs with hereditary footpad hyperkeratosis (HFH)
- PMID: 24832243
- PMCID: PMC4022470
- DOI: 10.1371/journal.pgen.1004370
A mutation in the FAM83G gene in dogs with hereditary footpad hyperkeratosis (HFH)
Abstract
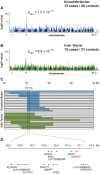
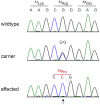
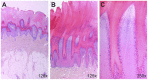
Hereditary footpad hyperkeratosis (HFH) represents a palmoplantar hyperkeratosis, which is inherited as a monogenic autosomal recessive trait in several dog breeds, such as e.g. Kromfohrländer and Irish Terriers. We performed genome-wide association studies (GWAS) in both breeds. In Kromfohrländer we obtained a single strong association signal on chromosome 5 (p(raw) = 1.0×10(-13)) using 13 HFH cases and 29 controls. The association signal replicated in an independent cohort of Irish Terriers with 10 cases and 21 controls (p(raw) = 6.9×10(-10)). The analysis of shared haplotypes among the combined Kromfohrländer and Irish Terrier cases defined a critical interval of 611 kb with 13 predicted genes. We re-sequenced the genome of one affected Kromfohrländer at 23.5× coverage. The comparison of the sequence data with 46 genomes of non-affected dogs from other breeds revealed a single private non-synonymous variant in the critical interval with respect to the reference genome assembly. The variant is a missense variant (c.155G>C) in the FAM83G gene encoding a protein with largely unknown function. It is predicted to change an evolutionary conserved arginine into a proline residue (p.R52P). We genotyped this variant in a larger cohort of dogs and found perfect association with the HFH phenotype. We further studied the clinical and histopathological alterations in the epidermis in vivo. Affected dogs show a moderate to severe orthokeratotic hyperplasia of the palmoplantar epidermis. Thus, our data provide the first evidence that FAM83G has an essential role for maintaining the integrity of the palmoplantar epidermis.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: The authors CK, CDdC and AT are employees of Antagene, a private company selling diagnostic tests in dogs.
Figures


References
-
- Reis A, Hennies HC, Langbein L, Digweed M, Mischke D, et al. (1994) Keratin 9 gene mutations in epidermolytic palmoplantar keratoderma (EPPK). Nat Genet 6: 174–179. - PubMed
-
- Fu DJ, Thomson C, Lunny DP, Dopping-Hepenstal PJ, McGrath JA, et al. (2013) Keratin 9 is required for the structural integrity and terminal differentiation of the palmoplantar epidermis. J Invest Dermatol 134: 754–63 doi: 10.1038/jid.2013.356 - DOI - PMC - PubMed
-
- Kimonis V, DiGiovanna JJ, Yang JM, Doyle SZ, Bale SJ, et al. (1994) A mutation in the V1 end domain of keratin 1 in non-epidermolytic palmar-plantar keratoderma. J Invest Dermatol 103: 764–769. - PubMed
-
- Morais P, Mota A, Baudrier T, Lopes JM, Cerqueira R, et al. (2009) Epidermolytic hyperkeratosis with palmoplantar keratoderma in a patient with KRT10 mutation. Eur J Dermatol 19: 333–336. - PubMed
-
- McLean WH, Rugg EL, Lunny DP, Morley SM, Lane EB, et al. (1995) Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet 9: 273–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

