PET radiotracer [¹⁸F]-P6 selectively targeting COX-1 as a novel biomarker in ovarian cancer: preliminary investigation
- PMID: 24832612
- PMCID: PMC4401082
- DOI: 10.1016/j.ejmech.2014.04.074
PET radiotracer [¹⁸F]-P6 selectively targeting COX-1 as a novel biomarker in ovarian cancer: preliminary investigation
Abstract
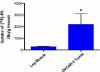
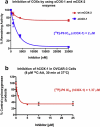
Cyclooxygenase-1 (COX-1), but not COX-2, is expressed at high levels in the early stages of human epithelial ovarian cancer where it seems to play a key role in cancer onset and progression. As a consequence, COX-1 is an ideal biomarker for early ovarian cancer detection. A series of novel fluorinated COX-1-targeted imaging agents derived from P6 was developed by using a highly selective COX-1 inhibitor as a lead compound. Among these new compounds, designed by structural modification of P6, 3-(5-chlorofuran-2-yl)-5-(fluoromethyl)-4-phenylisoxazole ([(18/19)F]-P6) is the most promising derivative [IC50 = 2.0 μM (purified oCOX-1) and 1.37 μM (hOVCAR-3 cell COX-1)]. Its tosylate precursor was also prepared and, a method for radio[(18)F]chemistry was developed and optimized. The radiochemistry was carried out using a carrier-free K(18)F/Kryptofix 2.2.2 complex, that afforded [(18)F]-P6 in good radiochemical yield (18%) and high purity (>95%). In vivo PET/CT imaging data showed that the radiotracer [(18)F]-P6 was selectively taken up by COX-1-expressing ovarian carcinoma (OVCAR 3) tumor xenografts as compared with the normal leg muscle. Our results suggest that [(18)F]-P6 might be an useful radiotracer in preclinical and clinical settings for in vivo PET-CT imaging of tissues that express elevated levels of COX-1.
Keywords: Biomarker; Cyclooxygenase (COX)-1; OVCAR-3 cell; Ovarian cancer; PET-CT imaging; [(18)F]-P6 radiotracer.
Copyright © 2014 Elsevier Masson SAS. All rights reserved.
Figures




References
-
- Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. Journal of Medicinal Chemistry. 2007;50:1425–1441. - PubMed
-
- Perrone MG, Scilimati A, Simone L, Vitale P. Selective COX-1 inhibition: a therapeutic target to be reconsidered. Current Medicinal Chemistry. 2010;17:3769–3805. - PubMed
-
- Radi ZA, Khan NK. Effect of cyclooxygenase inhibition on the gastrointestinal tract. Experimental and Toxicologic Pathology. 2006;58:163–173. - PubMed
-
- Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and meta-static potential of tumor cells. Biochemical and Biophysical Research Communications. 2002;299:886–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

