Adjuvant chemotherapy for advanced endometrial cancer
- PMID: 24832785
- PMCID: PMC6457820
- DOI: 10.1002/14651858.CD010681.pub2
Adjuvant chemotherapy for advanced endometrial cancer
Abstract
Background: Approximately 13% of women diagnosed with endometrial cancer present with advanced stage disease (International Federation of Gynecology and Obstetrics (FIGO) stage III/IV). The standard treatment of advanced endometrial cancer consists of cytoreductive surgery followed by radiation therapy, or chemotherapy, or both. There is currently little agreement about which adjuvant treatment is the safest and most effective.
Objectives: To evaluate the effectiveness and safety of adjuvant chemotherapy compared with radiotherapy or chemoradiation, and to determine which chemotherapy agents are most effective in women presenting with advanced endometrial cancer (FIGO stage III/IV).
Search methods: We searched the Cochrane Gynaecological Cancer Collaborative Review Group's Trial Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 10 2013), MEDLINE and EMBASE up to November 2013. Also we searched electronic clinical trial registries for ongoing trials.
Selection criteria: Randomised controlled trials (RCTs) of adjuvant chemotherapy compared with radiotherapy or chemoradiation in women with FIGO stage III and IV endometrial cancer.
Data collection and analysis: Two review authors selected trials, extracted data, and assessed trials for risk of bias. Where necessary, we contacted trial investigators for relevant, unpublished data. We pooled data using the random-effects model in Review Manager (RevMan) software.
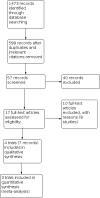
Main results: We included four multicentre RCTs involving 1269 women with primary FIGO stage III/IV endometrial cancer. We considered the trials to be at low to moderate risk of bias. All participants received primary cytoreductive surgery. Two trials, evaluating 620 women (83% stage III, 17% stage IV), compared adjuvant chemotherapy with adjuvant radiotherapy; one trial evaluating 552 women (88% stage III, 12% stage IV) compared two chemotherapy regimens (cisplatin/doxorubicin/paclitaxel (CDP) versus cisplatin/doxorubicin (CD) treatment) in women who had all undergone adjuvant radiotherapy; and one trial contributed no data.Overall survival (OS) and progression-free survival (PFS) was longer with adjuvant chemotherapy compared with adjuvant radiotherapy (OS: hazard ratio (HR) 0.75, 95% confidence interval (CI) 0.57 to 0.99, I² = 22%; and PFS: HR 0.74, 95% CI 0.59 to 0.92, I² = 0%). Sensitivity analysis using adjusted and unadjusted OS data, gave similar results. In subgroup analyses, the effects on survival in favour of chemotherapy were not different for stage III and IV, or stage IIIA and IIIC (tests for subgroup differences were not significant and I² = 0%). This evidence was of moderate quality. Data from one trial showed that women receiving adjuvant chemotherapy were more likely to experience haematological and neurological adverse events and alopecia, and more likely to discontinue treatment (33/194 versus 6/202; RR 5.73, 95% CI 2.45 to 13.36), than those receiving adjuvant radiotherapy. There was no statistically significant difference in treatment-related deaths between the chemotherapy and radiotherapy treatment arms (8/309 versus 5/311; Risk Ratio (RR) 1.67, 95% CI 0.55 to 5.00).There was no clear difference in PFS between intervention groups in the one trial that compared CDP versus CD (552 women; HR 0.90, 95% CI 0.69 to 1.17). We considered this evidence to be of moderate quality. Mature OS data from this trial were not yet available. Severe haematological and neurological adverse events occurred more frequently with CDP than CD.We found no trials to include of adjuvant chemotherapy versus chemoradiation in advanced endometrial cancer; however we identified one ongoing trial of this comparison.
Authors' conclusions: There is moderate quality evidence that chemotherapy increases survival time after primary surgery by approximately 25% relative to radiotherapy in stage III and IV endometrial cancer. There is limited evidence that it is associated with more adverse effects. There is some uncertainty as to whether triplet regimens offer similar survival benefits over doublet regimens in the long-term. Further research is needed to determine which chemotherapy regimen(s) are the most effective and least toxic, and whether the addition of radiotherapy further improves outcomes. A large trial evaluating the benefits and risks of adjuvant chemoradiation versus chemotherapy in advanced endometrial cancer is ongoing.
Conflict of interest statement
None
Figures



















Update of
References
References to studies included in this review
GOG 122 {published data only}
-
- Bruner DW, Barsevick A, Tian C, Randall M, Mannel R, Cohn DE, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Quality of Life Research 2007;16(1):89-100. - PubMed
-
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006;24(1):36-44. - PubMed
-
- Tewari KS, Filiaci VL, Spirtos NM, Mannel RS, Thigpen JT, Cibull ML, et al. Association of number of positive nodes and cervical stroma invasion with outcome of advanced endometrial cancer treated with chemotherapy or whole abdominal irradiation: a Gynecologic Oncology Group study. Gynecologic Oncology 2012;125(1):87-93. - PubMed
GOG 184 {published data only}
-
- Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecologic Oncology 2009;112(3):543-52. - PMC - PubMed
Maggi 2006 {published data only}
Susumu 2008 {published data only}
-
- Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology 2008;108(1):226-33. - PubMed
References to studies excluded from this review
Aapro 2003 {published data only}
-
- Aapro MS, Wijk FH, Bolis G, Chevallier B, Burg ME, Poveda A, et al. Doxurubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Annals of Oncology 2003;14(3):441-8. - PubMed
Fleming 2004a {published data only}
-
- Fleming GF, Filiaci VL, Bentley RC, Herzog T, Sorosky J, Vaccarello L, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Annals of Oncology 2004;15(8):1173-8. - PubMed
Fleming 2004b {published data only}
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159-66. - PubMed
Gallion 2003 {published data only}
-
- Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2003;21(20):3808-13. - PubMed
Hogberg 2010 {published data only}
-
- Hogberg T, Rosenberg P, Kristensen G, Oliveira CF, Pont Christensen R, Sorbe B, et al. A randomized phase III study on adjuvant treatment with radiation +/- chemotherapy in early-stage high-risk endometrial cancer (NSGO-EC-9501/EORTC55991) [Abstract]. Journal of Clinical Oncology. Abstracts of the 43rd Annual Meeting of the American Society of Clinical Oncology 2007;25(18 Suppl):Abstract 5503.
Kuoppala 2008 {published data only}
-
- Kuoppala T, Mäenpää J, Tomas E, Puistola U, Salmi T, Grenman S, et al. Surgically staged high-risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecologic Oncology 2008;110(2):190-5. - PubMed
Mustea 2013 {published data only}
-
- Mustea A, Koensgen D, Belau A, Sehouli J, Lichtenegger W, Schneidewind L, et al. Adjuvant sequential chemoradiation therapy in high-risk endometrial cancer: results of a prospective, multicenter phase-II study of the NOGGO (North-Eastern German Society of Gynaecological Oncology). Cancer Chemotherapy and Pharmacology 2013;72(5):975-83. - PubMed
Nomura 2011 {published data only}
-
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Annals of Oncology 2011;22(3):636-42. - PubMed
Wolfson 2007 {published data only}
-
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee Y-C, Futoran RJ et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology 2007;107(2):177-185. - PMC - PubMed
References to ongoing studies
GOG 0258 {published data only}
-
- Gynecologic Oncology Group. Carboplatin and paclitaxel with or without cisplatin and radiation therapy in treating patients with stage I, stage II, stage III, or stage IVA endometrial cancer. http://www.controlled-trials.com/mrct/trial/587551/endometrial+cancer (accessed 11 November 2013).
PORTEC‐3 {unpublished data only}
-
- PORTEC-3 collaborators. Chemotherapy and radiation therapy compared with radiation therapy alone in treating patients with high-risk stage I, stage II, or stage III endometrial cancer. http://www.controlled-trials.com/mrct/trial/386235/PORTEC-3 2012.
Additional references
Barlin 2010
-
- Barlin JN, Puri I, Bristow RE. Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecologic Oncology 2010;118(1):14-8. - PubMed
Berek 2010
-
- Berek JS, Hacker NF. Berek and Hacker's Gynecologic Oncology. 5th edition. Lippincott Williams & Wilkins, 2010.
Colombo 2011
-
- Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2011;22(Suppl 6):vi35-39. - PubMed
Creasman 2006
-
- Creasman WT, Odicino F, Mausinneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International Journal of Gynaecology and Obstetrics 2006;95(Suppl 1):S105-43. - PubMed
CTCAE 2006
-
- Common terminology criteria for adverse events v3.0 (CTCAE). http://ctep.cancer.gov/forms/CTCAEv3.pdf 2006.
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: In: Egger M, Davey Smith G, Altman DG, editor(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001.
Dubinsky 2004
-
- Dubinsky TJ. Value of sonography in the diagnosis of abnormal vaginal bleeding. Journal of Clinical Ultrasound 2004;32(7):348–53. - PubMed
ENDNOTE X7.0.2 [Computer program]
-
- ENDNOTE X7.0.2. Thomson Reuters, 2013.
Evans 2011
Galaal 2013
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Humber 2007
-
- Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane Collaboration. Annals of Oncology 2007;18(3):409–20. - PubMed
IARC 2013
-
- The International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx 2013.
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians 2008;58(2):71-96. - PubMed
Jemal 2011
-
- Jemal A, Bray F, Center MM , Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians 2011;61(2):69-90. - PubMed
Johnson 2011
Kokka 2010
Martin‐Hirsch 2011
McMeekin 2009
-
- McMeekin DS. Where is the future of endometrial cancer therapy? Annals of Oncology 2009;20(11):1757-61. - PubMed
Review Manager (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
SEER 2007
-
- Kosary CL. Chapter 15: Cancer of the corpus uteri. In: Gloeckler Ries LA, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors(s). Cancer survival among adults: US SEER program, 1988-2001. Patient and Tumor Characteristics. Bethesda, MD: National Cancer Institute, 2007:123-132.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

