The role of the bacterial flagellum in adhesion and virulence
- PMID: 24833223
- PMCID: PMC4009794
- DOI: 10.3390/biology2041242
The role of the bacterial flagellum in adhesion and virulence
Abstract
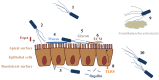
The bacterial flagellum is a complex apparatus assembled of more than 20 different proteins. The flagellar basal body traverses the cell wall, whereas the curved hook connects the basal body to the whip-like flagellar filament that protrudes several µm from the bacterial cell. The flagellum has traditionally been regarded only as a motility organelle, but more recently it has become evident that flagella have a number of other biological functions. The major subunit, flagellin or FliC, of the flagellum plays a well-documented role in innate immunity and as a dominant antigen of the adaptive immune response. Importantly, flagella have also been reported to function as adhesins. Whole flagella have been indicated as significant in bacterial adhesion to and invasion into host cells. In various pathogens, e.g., Escherichia coli, Pseudomonas aeruginosa and Clostridium difficile, flagellin and/or the distally located flagellar cap protein have been reported to function as adhesins. Recently, FliC of Shiga-toxigenic E. coli was shown to be involved in cellular invasion via lipid rafts. Here, we examine the latest or most important findings regarding flagellar adhesive and invasive properties, especially focusing on the flagellum as a potential virulence factor.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

