Closed-loop brain-machine-body interfaces for noninvasive rehabilitation of movement disorders
- PMID: 24833254
- PMCID: PMC4099421
- DOI: 10.1007/s10439-014-1032-6
Closed-loop brain-machine-body interfaces for noninvasive rehabilitation of movement disorders
Abstract
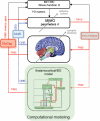
Traditional approaches for neurological rehabilitation of patients affected with movement disorders, such as Parkinson's disease (PD), dystonia, and essential tremor (ET) consist mainly of oral medication, physical therapy, and botulinum toxin injections. Recently, the more invasive method of deep brain stimulation (DBS) showed significant improvement of the physical symptoms associated with these disorders. In the past several years, the adoption of feedback control theory helped DBS protocols to take into account the progressive and dynamic nature of these neurological movement disorders that had largely been ignored so far. As a result, a more efficient and effective management of PD cardinal symptoms has emerged. In this paper, we review closed-loop systems for rehabilitation of movement disorders, focusing on PD, for which several invasive and noninvasive methods have been developed during the last decade, reducing the complications and side effects associated with traditional rehabilitation approaches and paving the way for tailored individual therapeutics. We then present a novel, transformative, noninvasive closed-loop framework based on force neurofeedback and discuss several future developments of closed-loop systems that might bring us closer to individualized solutions for neurological rehabilitation of movement disorders.
Figures




Similar articles
-
Approaches to closed-loop deep brain stimulation for movement disorders.Neurosurg Focus. 2018 Aug;45(2):E2. doi: 10.3171/2018.5.FOCUS18173. Neurosurg Focus. 2018. PMID: 30064321
-
Deep Brain Stimulation in Movement Disorders: From Experimental Surgery to Evidence-Based Therapy.Mov Disord. 2019 Dec;34(12):1795-1810. doi: 10.1002/mds.27860. Epub 2019 Oct 3. Mov Disord. 2019. PMID: 31580535 Review.
-
Toward Electrophysiology-Based Intelligent Adaptive Deep Brain Stimulation for Movement Disorders.Neurotherapeutics. 2019 Jan;16(1):105-118. doi: 10.1007/s13311-018-00705-0. Neurotherapeutics. 2019. PMID: 30607748 Free PMC article. Review.
-
Deep brain stimulation for movement disorders: update on recent discoveries and outlook on future developments.J Neurol. 2015 Nov;262(11):2583-95. doi: 10.1007/s00415-015-7790-8. Epub 2015 Jun 3. J Neurol. 2015. PMID: 26037016 Review.
-
Deep brain stimulation for movement disorders.Neurotherapeutics. 2014 Jul;11(3):465-74. doi: 10.1007/s13311-014-0274-1. Neurotherapeutics. 2014. PMID: 24833244 Free PMC article. Review.
Cited by
-
Electroencephalographic read-outs of the modulation of cortical network activity by deep brain stimulation.Bioelectron Med. 2018 Mar 15;4:2. doi: 10.1186/s42234-018-0003-x. eCollection 2018. Bioelectron Med. 2018. PMID: 32232078 Free PMC article. Review.
-
G-Exos: A wearable gait exoskeleton for walk assistance.Front Neurorobot. 2022 Nov 10;16:939241. doi: 10.3389/fnbot.2022.939241. eCollection 2022. Front Neurorobot. 2022. PMID: 36439287 Free PMC article.
-
Brain structural and functional correlates to defense-related inhibition of muscle sympathetic nerve activity in man.Sci Rep. 2022 Feb 7;12(1):1990. doi: 10.1038/s41598-022-05910-8. Sci Rep. 2022. PMID: 35132113 Free PMC article.
-
Wearable EEG and beyond.Biomed Eng Lett. 2019 Jan 4;9(1):53-71. doi: 10.1007/s13534-018-00093-6. eCollection 2019 Feb. Biomed Eng Lett. 2019. PMID: 30956880 Free PMC article. Review.
-
Bio-inspired benchmark generator for extracellular multi-unit recordings.Sci Rep. 2017 Feb 24;7:43253. doi: 10.1038/srep43253. Sci Rep. 2017. PMID: 28233819 Free PMC article.
References
-
- Lyons KE, Pahwa R. Pharmacotherapy of essential tremor: an overview of existing and upcoming agents. CNS Drug. 2008;22:1037–1045. - PubMed
-
- Thenganatt MA, Fahn S. Botulinum toxin for the treatment of movement disorders. Curr. Neurol. Neurosci. 2012;12:399–409. - PubMed
-
- Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson's disease therapy. Ann. Neurol. 2003;53(Supp.3):S3–S12. - PubMed
-
- Raja M, Bentivoglio AR. Impulsive and compulsive behaviors during dopamine replacement treatment in Parkinson's disease and other disorders. Curr. Drug Saf. 2012;7:63–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

