High-throughput screen of natural product libraries for hsp90 inhibitors
- PMID: 24833337
- PMCID: PMC4009755
- DOI: 10.3390/biology3010101
High-throughput screen of natural product libraries for hsp90 inhibitors
Abstract
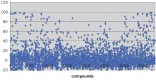
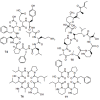
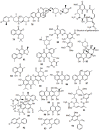
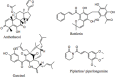
Hsp90 has become the target of intensive investigation, as inhibition of its function has the ability to simultaneously incapacitate proteins that function in pathways that represent the six hallmarks of cancer. While a number of Hsp90 inhibitors have made it into clinical trials, a number of short-comings have been noted, such that the search continues for novel Hsp90 inhibitors with superior pharmacological properties. To identify new potential Hsp90 inhibitors, we have utilized a high-throughput assay based on measuring Hsp90-dependent refolding of thermally denatured luciferase to screen natural compound libraries. Over 4,000 compounds were screen with over 100 hits. Data mining of the literature indicated that 51 compounds had physiological effects that Hsp90 inhibitors also exhibit, and/or the ability to downregulate the expression levels of Hsp90-dependent proteins. Of these 51 compounds, seven were previously characterized as Hsp90 inhibitors. Four compounds, anthothecol, garcinol, piplartine, and rottlerin, were further characterized, and the ability of these compounds to inhibit the refolding of luciferase, and reduce the rate of growth of MCF7 breast cancer cells, correlated with their ability to suppress the Hsp90-dependent maturation of the heme-regulated eIF2α kinase, and deplete cultured cells of Hsp90-dependent client proteins. Thus, this screen has identified an additional 44 compounds with known beneficial pharmacological properties, but with unknown mechanisms of action as possible new inhibitors of the Hsp90 chaperone machine.
Figures



References
-
- Whitesell L., Mimnaugh E.G., de Costa B., Myers C.E., Neckers L.M. Inhibition of heat shock protein hsp90-pp60v-Src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA. 1994;91:8324–8328. - PMC - PubMed
-
- Grenert J.P., Sullivan W.P., Fadden P., Haystead T.A., Clark J., Mimnaugh E., Krutzsch H., Ochel H.J., Schulte T.W., Sausville E., et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 1997;272:23843–23850. - PubMed
-
- Sharma S.V., Agatsuma T., Nakano H. Targeting of the protein chaperone, Hsp90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–2645. - PubMed
-
- Schulte T.W., Akinaga S., Soga S., Sullivan W., Stensgard B., Toft D., Neckers L.M. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:ARBTTN>2.3.CO;2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

