Stable enhanced green fluorescent protein expression after differentiation and transplantation of reporter human induced pluripotent stem cells generated by AAVS1 transcription activator-like effector nucleases
- PMID: 24833591
- PMCID: PMC4073825
- DOI: 10.5966/sctm.2013-0212
Stable enhanced green fluorescent protein expression after differentiation and transplantation of reporter human induced pluripotent stem cells generated by AAVS1 transcription activator-like effector nucleases
Abstract
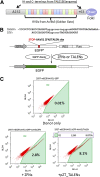
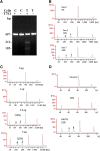
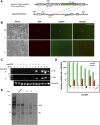
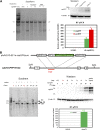
Human induced pluripotent stem (hiPS) cell lines with tissue-specific or ubiquitous reporter genes are extremely useful for optimizing in vitro differentiation conditions as well as for monitoring transplanted cells in vivo. The adeno-associated virus integration site 1 (AAVS1) locus has been used as a "safe harbor" locus for inserting transgenes because of its open chromatin structure, which permits transgene expression without insertional mutagenesis. However, it is not clear whether targeted transgene expression at the AAVS1 locus is always protected from silencing when driven by various promoters, especially after differentiation and transplantation from hiPS cells. In this paper, we describe a pair of transcription activator-like effector nucleases (TALENs) that enable more efficient genome editing than the commercially available zinc finger nuclease at the AAVS1 site. Using these TALENs for targeted gene addition, we find that the cytomegalovirus-immediate early enhancer/chicken β-actin/rabbit β-globin (CAG) promoter is better than cytomegalovirus 7 and elongation factor 1α short promoters in driving strong expression of the transgene. The two independent AAVS1, CAG, and enhanced green fluorescent protein (EGFP) hiPS cell reporter lines that we have developed do not show silencing of EGFP either in undifferentiated hiPS cells or in randomly and lineage-specifically differentiated cells or in teratomas. Transplanting cardiomyocytes from an engineered AAVS1-CAG-EGFP hiPS cell line in a myocardial infarcted mouse model showed persistent expression of the transgene for at least 7 weeks in vivo. Our results show that high-efficiency targeting can be obtained with open-source TALENs and that careful optimization of the reporter and transgene constructs results in stable and persistent expression in vitro and in vivo.
Keywords: AAVS1; Differentiation; Genome editing; Human induced pluripotent stem cells; Transcription activator-like effector nuclease (TALEN); Transplantation.
©AlphaMed Press.
Figures


References
-
- Walia B, Satija N, Tripathi RP, et al. Induced pluripotent stem cells: Fundamentals and applications of the reprogramming process and its ramifications on regenerative medicine. Stem Cell Rev. 2012;8:100–115. - PubMed
-
- Deacon T, Dinsmore J, Costantini LC, et al. Blastula-stage stem cells can differentiate into dopaminergic and serotonergic neurons after transplantation. Exp Neurol. 1998;149:28–41. - PubMed
-
- Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007;25:24–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

