Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy
- PMID: 24833701
- PMCID: PMC4109489
- DOI: 10.1124/jpet.113.212076
Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy
Abstract
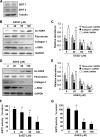
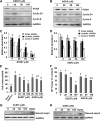
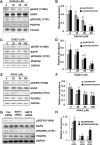
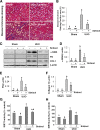
Our recent studies revealed that blocking class I/II histone deacetylases (HDACs) inhibits renal interstitial fibroblast activation and proliferation and alleviates development of renal fibrosis. However, the effect of class III HDAC, particularly sirtuin 1 and 2 (SIRT1 and SIRT2), inhibition on renal fibrogenesis remains elusive. Here, we demonstrate that both SIRT1 and SIRT2 were expressed in cultured renal interstitial fibroblasts (NRK-49F). Exposure of NRK-49F to sirtinol, a selective inhibitor of SIRT1/2, or EX527 (6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide), an inhibitor for SIRT1, resulted in reduced expression of fibroblast activation markers (α-smooth muscle actin, fibronectin, and collagen I) as well as proliferation markers (proliferating cell nuclear antigen, cyclin D1, cyclin E) in dose- and time-dependent manners. Treatment with a SIRT2 inhibitor, AGK2 (2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide), also dose- and time-dependently inhibited renal fibroblast activation and, to a lesser extent, cell proliferation. Furthermore, silencing of either SIRT1 or SIRT2 by small interfering RNA exhibited similar inhibitory effects. In a mouse model of obstructive nephropathy, administration of sirtinol attenuated deposition of collagen fibrils as well as reduced expression of α-smooth muscle actin, collagen I, and fibronectin in the injured kidney. SIRT1/2 inhibition-mediated antifibrotic effects are associated with dephosphorylation of epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor-β (PDGFRβ), and signal transducer and activator of transcription 3. Thus, SIRT1/2 activity may contribute to renal fibroblast activation and proliferation as well as renal fibrogenesis through activation of at least EGFR and PDGFRβ signaling. Blocking SIRT1/2 activation may have therapeutic potential for the treatment of chronic kidney disease.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





Similar articles
-
Activation of Sirtuin-1 Promotes Renal Fibroblast Activation and Aggravates Renal Fibrogenesis.J Pharmacol Exp Ther. 2015 Aug;354(2):142-51. doi: 10.1124/jpet.115.224386. Epub 2015 May 28. J Pharmacol Exp Ther. 2015. PMID: 26022003 Free PMC article.
-
Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling.PLoS One. 2013;8(1):e54001. doi: 10.1371/journal.pone.0054001. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342059 Free PMC article.
-
Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy.Am J Physiol Renal Physiol. 2009 Oct;297(4):F996-F1005. doi: 10.1152/ajprenal.00282.2009. Epub 2009 Jul 29. Am J Physiol Renal Physiol. 2009. PMID: 19640900 Free PMC article.
-
Inhibition of SIRT2 Alleviates Fibroblast Activation and Renal Tubulointerstitial Fibrosis via MDM2.Cell Physiol Biochem. 2018;46(2):451-460. doi: 10.1159/000488613. Epub 2018 Mar 26. Cell Physiol Biochem. 2018. PMID: 29614506
-
Sirtuin inhibitors, EX527 and AGK2, suppress cell migration by inhibiting HSF1 protein stability.Oncol Rep. 2016 Jan;35(1):235-42. doi: 10.3892/or.2015.4381. Epub 2015 Nov 2. Oncol Rep. 2016. PMID: 26530275
Cited by
-
The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor β Signaling.Arthritis Rheumatol. 2015 May;67(5):1323-34. doi: 10.1002/art.39061. Arthritis Rheumatol. 2015. PMID: 25707573 Free PMC article.
-
EX-527 Prevents the Progression of High-Fat Diet-Induced Hepatic Steatosis and Fibrosis by Upregulating SIRT4 in Zucker Rats.Cells. 2020 Apr 29;9(5):1101. doi: 10.3390/cells9051101. Cells. 2020. PMID: 32365537 Free PMC article.
-
The role of Sirtuin 1 in regulation of fibrotic genes expression in pre-adipocytes.J Diabetes Metab Disord. 2024 Mar 7;23(1):1081-1091. doi: 10.1007/s40200-024-01389-4. eCollection 2024 Jun. J Diabetes Metab Disord. 2024. PMID: 38932833 Free PMC article.
-
Protective Effects of SIRT2 Inhibition on Cardiac Fibrosis.Anatol J Cardiol. 2025 Jan 31;29(4):173-80. doi: 10.14744/AnatolJCardiol.2025.4770. Online ahead of print. Anatol J Cardiol. 2025. PMID: 39885712 Free PMC article.
-
SIRT2 regulates oxidative stress-induced cell death through deacetylation of c-Jun NH2-terminal kinase.Cell Death Differ. 2018 Sep;25(9):1638-1656. doi: 10.1038/s41418-018-0069-8. Epub 2018 Feb 15. Cell Death Differ. 2018. PMID: 29449643 Free PMC article.
References
-
- Akgedik R, Akgedik S, Karamanlı H, Uysal S, Bozkurt B, Ozol D, Armutcu F, Yıldırım Z. (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35:1732–1741 - PubMed
-
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. (2009) Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des 74:619–624 - PubMed
-
- Bonner JC. (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255–273 - PubMed
-
- Catania A, Iavarone C, Carlomagno SM, Chiariello M. (2006) Selective transcription and cellular proliferation induced by PDGF require histone deacetylase activity. Biochem Biophys Res Commun 343:544–554 - PubMed
-
- Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. (2013) The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int 83:404–413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

