The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice
- PMID: 24833737
- PMCID: PMC4059081
- DOI: 10.1167/iovs.13-13699
The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice
Abstract
Purpose: To determine the connections between the ciliary muscle (CM), trabecular meshwork (TM), and Schlemm's canal (SC) and their innervations that allows CM contraction (by pilocarpine) to influence conventional outflow in mice.
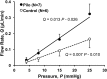
Methods: Sequential sections and whole mounts of murine corneoscleral angles were stained for elastin, α-smooth muscle actin (αSMA), vesicular acetylcholine transporter (VAChT), neuronal nitric oxide synthase (nNOS), vasoactive intestinal peptide (VIP), and tyrosine hydroxylase (TH). Elastic (EL) fibers between the CM, TM, and SC were examined in ultrathin, sequential sections from different planes. The effect of pilocarpine (100 μM) on conventional outflow facility was measured by perfusion of enucleated mouse eyes.
Results: The mouse TM contains a three-dimensional (3D) net of EL fibers connecting the inner wall of SC to the cornea anteriorly, the ciliary body (CB) internally and the choroid and CM posteriorly. The CM bifurcates near the posterior TM, extending outer tendons to the juxtacanalicular tissue and inner wall of SC and internal connections to the lamellated TM and CB. Ciliary muscle and lamellated TM cells stain with αSMA and are innervated by VAChT-containing nerve fibers, without TH, VIP, or nNOS. Pilocarpine doubled outflow facility.
Conclusions: Mouse eyes resemble primate eyes not only by their well developed SC and TM, but also by their 3D EL net tethering together the TM and SC inner wall and by the tendinous insertion of the CM into this net. The increase in outflow facility following cholinergic stimulation in mice, as in primates, supports using mice for studies of aqueous humor dynamics and glaucoma.
Keywords: aqueous humor; ciliary muscle; glaucoma; pilocarpine; trabecular meshwork.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures






References
-
- Bárány EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus ethiops). Invest Ophthalmol Vis Sci. 1962; 1: 712–727 - PubMed
-
- Bárány EH, Rohen JW. Localized contraction and relaxation within the ciliary muscle of the vervet monkey (Cercopithecus ethiops). In: Rohen JW. ed The Structure of the Eye, Second Symposium. Stuttgart, Germany: FK Schattauer Verlag; 1965: 287–311
-
- Rohen JW, Lütjen E, Bárány E. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. A study in two contrasting monkey species, Macaca irus and Cercopithecus aethiops. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1967; 172: 23–47 - PubMed
-
- Rohen JW, Futa R, Lütjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981; 21: 574–585 - PubMed
-
- Lütjen-Drecoll E. Structural factors influencing outflow facility and its changeability under drugs. A study in Macaca arctoides. Invest Ophthalmol. 1973; 12: 280–294 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

