Sniff adjustment in an odor discrimination task in the rat: analytical or synthetic strategy?
- PMID: 24834032
- PMCID: PMC4017146
- DOI: 10.3389/fnbeh.2014.00145
Sniff adjustment in an odor discrimination task in the rat: analytical or synthetic strategy?
Abstract
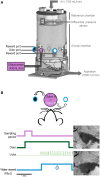
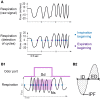
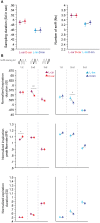
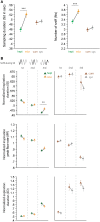
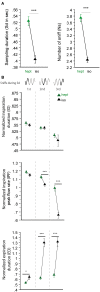
A growing body of evidence suggests that sniffing is not only the mode of delivery for odorant molecules but also contributes to olfactory perception. However, the precise role of sniffing variations remains unknown. The zonation hypothesis suggests that animals use sniffing variations to optimize the deposition of odorant molecules on the most receptive areas of the olfactory epithelium (OE). Sniffing would thus depend on the physicochemical properties of odorants, particularly their sorption. Rojas-Líbano and Kay (2012) tested this hypothesis and showed that rats used different sniff strategies when they had to target a high-sorption (HS) molecule or a low-sorption (LS) molecule in a binary mixture. Which sniffing strategy is used by rats when they are confronted to discrimination between two similarly sorbent odorants remains unanswered. Particularly, is sniffing adjusted independently for each odorant according to its sorption properties (analytical processing), or is sniffing adjusted based on the pairing context (synthetic processing)? We tested these hypotheses on rats performing a two-alternative choice discrimination of odorants with similar sorption properties. We recorded sniffing in a non-invasive manner using whole-body plethysmography during the behavioral task. We found that sniffing variations were not only a matter of odorant sorption properties and that the same odorant was sniffed differently depending on the odor pair in which it was presented. These results suggest that rather than being adjusted analytically, sniffing is instead adjusted synthetically and depends on the pair of odorants presented during the discrimination task. Our results show that sniffing is a specific sensorimotor act that depends on complex synthetic processes.
Keywords: discrimination; olfaction; olfactomotor act; rat; sniffing; sorption properties.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

