Clinical implications of BRAF mutation test in colorectal cancer
- PMID: 24834238
- PMCID: PMC4017487
Clinical implications of BRAF mutation test in colorectal cancer
Abstract
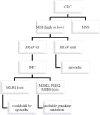
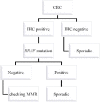
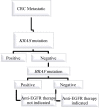
Knowledge about the clinical significance of V-Raf Murine Sarcoma Viral Oncogene Homolog B1 (BRAF) mutations in colorectal cancer (CRC) is growing. BRAF encodes a protein kinase involved with intracellular signaling and cell division. The gene product is a downstream effector of Kirsten Ras 1(KRAS) within the RAS/RAF/MAPK cellular signaling pathway. Evidence suggests that BRAF mutations, like KRAS mutations, result in uncontrolled, non-growth factor-dependent cellular proliferation. Similar to the rationale that KRAS mutation precludes effective treatment with anti-EGFR drugs. Recently, BRAF mutation testing has been introduced into routine clinical laboratories because its significance has become clearer in terms of effect on pathogenesis of CRC, utility in differentiating sporadic CRC from Lynch syndrome (LS), prognosis, and potential for predicting patient outcome in response to targeted drug therapy. In this review we describe the impact of BRAF mutations for these aspects.
Keywords: BRAF mutation; Colorectal Cancer; Prognosis value.
Figures
References
-
- Jass JR. Colorectal cancer: a multipathway disease. Crit Rev Oncog. 2006;12:273–87. - PubMed
-
- Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30. - PubMed
-
- Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012;55:128–33. - PubMed
-
- Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27:1423–31. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
