Deficiency in steroid receptor coactivator 3 enhances cytokine production in IgE-stimulated mast cells and passive systemic anaphylaxis in mice
- PMID: 24834318
- PMCID: PMC4021842
- DOI: 10.1186/2045-3701-4-21
Deficiency in steroid receptor coactivator 3 enhances cytokine production in IgE-stimulated mast cells and passive systemic anaphylaxis in mice
Abstract
Background: Steroid receptor coactivator 3 (SRC-3) is a multifunctional protein that plays an important role in malignancy of several cancers and in regulation of bacterial LPS-induced inflammation. However, the involvement of SRC-3 in allergic response remains unclear. Herein we used passive systemic anaphylaxis (PSA) and passive cutaneous anaphylaxis (PCA) mouse models to assess the role of SRC-3 in allergic response.
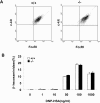
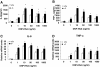
Results: SRC-3-deficient mice exhibited more severe allergic response as demonstrated by a significant drop in body temperature and a delayed recovery period compared to wild-type mice in PSA mouse model, whereas no significant difference was observed between two kinds of mice in PCA mouse models. Mast cells play a pivotal role in IgE-mediated allergic response. Antigen-induced aggregation of IgE receptor (FcϵRI) on the surface of mast cell activates a cascade of signaling events leading to the degranulation and cytokine production in mast cells. SRC-3-deficient bone marrow derived mast cells (BMMCs) developed normally but secreted more proinflammatory cytokines such as TNF-α and IL-6 than wild-type cells after antigen stimulation, whereas there was no significant difference in degranulation between two kinds of mast cells. Further studies showed that SRC-3 inhibited the activation of nuclear factor NF-κB pathway and MAPKs including extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 in antigen-stimulated mast cells.
Conclusions: Our data demonstrate that SRC-3 suppresses cytokine production in antigen-stimulated mast cells as well as PSA in mice at least in part through inhibiting NF-κB and MAPK signaling pathways. Therefore, SRC-3 plays a protective role in PSA and it may become a drug target for anaphylactic diseases.
Keywords: Mast cell; Passive cutaneous anaphylaxis; Passive systemic anaphylaxis; SRC-3.
Figures





Similar articles
-
Mycoepoxydiene inhibits antigen-stimulated activation of mast cells and suppresses IgE-mediated anaphylaxis in mice.Int Immunopharmacol. 2013 Oct;17(2):336-41. doi: 10.1016/j.intimp.2013.06.029. Epub 2013 Jul 13. Int Immunopharmacol. 2013. PMID: 23859869
-
AMP-activated protein kinase negatively regulates FcεRI-mediated mast cell signaling and anaphylaxis in mice.J Allergy Clin Immunol. 2013 Sep;132(3):729-736.e12. doi: 10.1016/j.jaci.2013.02.018. Epub 2013 Apr 12. J Allergy Clin Immunol. 2013. PMID: 23587332
-
WZ3146 inhibits mast cell Lyn and Fyn to reduce IgE-mediated allergic responses in vitro and in vivo.Toxicol Appl Pharmacol. 2019 Nov 15;383:114763. doi: 10.1016/j.taap.2019.114763. Epub 2019 Sep 14. Toxicol Appl Pharmacol. 2019. PMID: 31526816
-
A novel imidazo[1,5-b]isoquinolinone derivative, U63A05, inhibits Syk activation in mast cells to suppress IgE-mediated anaphylaxis in mice.J Pharmacol Sci. 2011;115(4):500-8. doi: 10.1254/jphs.10300fp. J Pharmacol Sci. 2011. PMID: 21498955
-
Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review.Antioxidants (Basel). 2024 Jul 29;13(8):920. doi: 10.3390/antiox13080920. Antioxidants (Basel). 2024. PMID: 39199166 Free PMC article. Review.
Cited by
-
Safranal Alleviated OVA-Induced Asthma Model and Inhibits Mast Cell Activation.Front Immunol. 2021 May 20;12:585595. doi: 10.3389/fimmu.2021.585595. eCollection 2021. Front Immunol. 2021. PMID: 34093515 Free PMC article.
References
-
- Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy. 2005;87:111–129. - PubMed
-
- Taketomi Y, Sunaga K, Tanaka S, Nakamura M, Arata S, Okuda T, Moon TC, Chang HW, Sugimoto Y, Kokame K, Myiata T, Murakami M, Kudo I. Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1-deficient mice. J Immunol. 2007;178:7042–7053. doi: 10.4049/jimmunol.178.11.7042. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

