Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline
- PMID: 24834977
- PMCID: PMC4171657
- DOI: 10.2337/db14-0047
Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline
Abstract
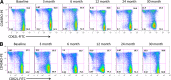
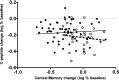
We previously reported that continuous 24-month costimulation blockade by abatacept significantly slows the decline of β-cell function after diagnosis of type 1 diabetes. In a mechanistic extension of that study, we evaluated peripheral blood immune cell subsets (CD4, CD8-naive, memory and activated subsets, myeloid and plasmacytoid dendritic cells, monocytes, B lymphocytes, CD4(+)CD25(high) regulatory T cells, and invariant NK T cells) by flow cytometry at baseline and 3, 6, 12, 24, and 30 months after treatment initiation to discover biomarkers of therapeutic effect. Using multivariable analysis and lagging of longitudinally measured variables, we made the novel observation in the placebo group that an increase in central memory (CM) CD4 T cells (CD4(+)CD45R0(+)CD62L(+)) during a preceding visit was significantly associated with C-peptide decline at the subsequent visit. These changes were significantly affected by abatacept treatment, which drove the peripheral contraction of CM CD4 T cells and the expansion of naive (CD45R0(-)CD62L(+)) CD4 T cells in association with a significantly slower rate of C-peptide decline. The findings show that the quantification of CM CD4 T cells can provide a surrogate immune marker for C-peptide decline after the diagnosis of type 1 diabetes and that costimulation blockade may exert its beneficial therapeutic effect via modulation of this subset.
Trial registration: ClinicalTrials.gov NCT00505375.
© 2014 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

References
-
- Stiller CR, Dupré J, Gent M, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 1984;223:1362–1367 - PubMed
-
- Harrison LC, Colman PG, Dean B, Baxter R, Martin FI. Increase in remission rate in newly diagnosed type I diabetic subjects treated with azathioprine. Diabetes 1985;34:1306–1308 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U01-DK-084565/DK/NIDDK NIH HHS/United States
- U01 DK085463/DK/NIDDK NIH HHS/United States
- UL1-RR-024139/RR/NCRR NIH HHS/United States
- UL1-RR-024153/RR/NCRR NIH HHS/United States
- U01-DK-061042/DK/NIDDK NIH HHS/United States
- U01 DK061036/DK/NIDDK NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK061041/DK/NIDDK NIH HHS/United States
- U01 DK085476/DK/NIDDK NIH HHS/United States
- UL1-RR-024975/RR/NCRR NIH HHS/United States
- M01 RR000400/RR/NCRR NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 DK061040/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR029890/RR/NCRR NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- UL1 RR031986/RR/NCRR NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01-DK-085509/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK085505/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- U01-DK-061036/DK/NIDDK NIH HHS/United States
- U01-DK-061041/DK/NIDDK NIH HHS/United States
- U01-DK-085463/DK/NIDDK NIH HHS/United States
- U01-DK-085466/DK/NIDDK NIH HHS/United States
- UL1-RR-031986/RR/NCRR NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- U01 DK061055/DK/NIDDK NIH HHS/United States
- U01-DK-085505/DK/NIDDK NIH HHS/United States
- 01-DK-061040/DK/NIDDK NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- U01-DK-061055/DK/NIDDK NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- UL1-RR-025744/RR/NCRR NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- UL1-RR-025780/RR/NCRR NIH HHS/United States
- UL1-RR-024982/RR/NCRR NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01-DK-061058/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- UL1-RR-025761/RR/NCRR NIH HHS/United States
- UL1-RR-029890/RR/NCRR NIH HHS/United States
- U01-DK-085461/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01-DK-061034/DK/NIDDK NIH HHS/United States
- UL1-RR-024131/RR/NCRR NIH HHS/United States
- U01-DK-061010/DK/NIDDK NIH HHS/United States
- M01-RR-00400/RR/NCRR NIH HHS/United States
- HHSN267200800019C/LM/NLM NIH HHS/United States
- U01-DK-085453/DK/NIDDK NIH HHS/United States
- U01-DK-061016/DK/NIDDK NIH HHS/United States
- U01-DK-085499/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

