How cells flow in the spreading of cellular aggregates
- PMID: 24835175
- PMCID: PMC4050549
- DOI: 10.1073/pnas.1323788111
How cells flow in the spreading of cellular aggregates
Abstract
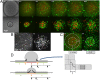
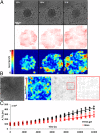
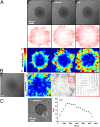
Like liquid droplets, cellular aggregates, also called "living droplets," spread onto adhesive surfaces. When deposited onto fibronectin-coated glass or polyacrylamide gels, they adhere and spread by protruding a cellular monolayer (precursor film) that expands around the droplet. The dynamics of spreading results from a balance between the pulling forces exerted by the highly motile cells at the periphery of the film, and friction forces associated with two types of cellular flows: (i) permeation, corresponding to the entry of the cells from the aggregates into the film; and (ii) slippage as the film expands. We characterize these flow fields within a spreading aggregate by using fluorescent tracking of individual cells and particle imaging velocimetry of cell populations. We find that permeation is limited to a narrow ring of width ξ (approximately a few cells) at the edge of the aggregate and regulates the dynamics of spreading. Furthermore, we find that the subsequent spreading of the monolayer depends heavily on the substrate rigidity. On rigid substrates, the migration of the cells in the monolayer is similar to the flow of a viscous liquid. By contrast, as the substrate gets softer, the film under tension becomes unstable with nucleation and growth of holes, flows are irregular, and cohesion decreases. Our results demonstrate that the mechanical properties of the environment influence the balance of forces that modulate collective cell migration, and therefore have important implications for the spreading behavior of tissues in both early development and cancer.
Keywords: tissue dynamics; tissue mechanosensitivity; wetting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

