Corticotrophin-Releasing Factor (CRF) and the urocortins are potent regulators of the inflammatory phenotype of human and mouse white adipocytes and the differentiation of mouse 3T3L1 pre-adipocytes
- PMID: 24835211
- PMCID: PMC4024041
- DOI: 10.1371/journal.pone.0097060
Corticotrophin-Releasing Factor (CRF) and the urocortins are potent regulators of the inflammatory phenotype of human and mouse white adipocytes and the differentiation of mouse 3T3L1 pre-adipocytes
Abstract
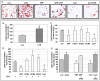
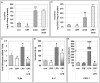
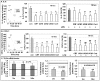
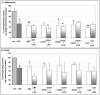
Chronic activation of innate immunity takes place in obesity and initiated by the hypertrophic adipocytes which obtain a pro-inflammatory phenotype. The corticotrophin-releasing factor (CRF) family of neuropeptides and their receptors (CRF1 and CRF2) affect stress response and innate immunity. Adipose tissue expresses a complete CRF system. The aim of this study was to examine the role of CRF neuropeptides in the immune phenotype of adipocytes assessed by their expression of the toll-like receptor-4 (TLR4), the production of inflammatory cytokines IL-6, TNF-α and IL-1β, chemokines IL-8, monocyte attractant protein-1 (MCP-1) and of the adipokines adiponectin, resistin and leptin. Our data are as follows: (a) CRF, UCN2 and UCN3 are expressed in human white adipocytes as well as CRFR1a, CRFR2a and CRFR2b but not CRFR2c. 3T3L1 pre-adipocytes and differentiated adipocytes expressed both CRF1 and CRF2 receptors and UCN3, while UCN2 was detected only in differentiated adipocytes. CRF2 was up-regulated in mouse mature adipocytes. (b) CRF1 agonists suppressed media- and LPS-induced pre-adipocyte differentiation while CRF2 receptor agonists had no effect. (c) In mouse pre-adipocytes, CRF2 agonists suppressed TLR4 expression and the production of IL-6, CXCL1 and adiponectin while CRF1 agonists had no effect. (d) In mature mouse adipocytes LPS induced IL-6 and CXCL1 production and suppressed leptin. (e) In human visceral adipocytes LPS induced IL-6, TNF-α, IL-8, MCP-1 and leptin production and suppressed adiponectin and resistin. (f) In mouse mature adipocytes CRF1 and CRF2 agonists suppressed basal and LPS-induced production of inflammatory cytokines, TLR4 expression and adiponectin production, while in human visceral adipocytes CRF and UCN1 suppressed basal and LPS-induced IL-6, TNF-α, IL-8 and MCP-1 production. In conclusion, the effects of the activation of CRF1 and CRF2 may be significant in ameliorating the pro-inflammatory activity of adipocytes in obesity.
Conflict of interest statement
Figures



References
-
- Tsatsanis C, Androulidaki A, Alissafi T, Charalampopoulos I, Dermitzaki E, et al. (2006) Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J Immunol 176: 1869–1877. - PubMed
-
- Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN (2007) Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-αlpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol 210: 774–783. - PubMed
-
- Charalampopoulos I, Androulidaki A, Minas V, Chatzaki E, Tsatsanis C, et al. (2006) Neuropeptide urocortin and its receptors are expressed in rat Kupffer cells. Neuroendocrinology 84: 49–57. - PubMed
-
- Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, et al. (2005) Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett 579: 4259–4264. - PubMed
-
- Trayhurn P, Wood IS (2005) Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 33: 1078–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

