Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in guinea pig
- PMID: 24835470
- PMCID: PMC4023991
- DOI: 10.1371/journal.pone.0097948
Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in guinea pig
Abstract
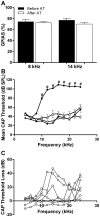
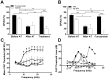
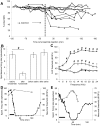
Cochlear trauma causes increased spontaneous activity (hyperactivity) to develop in central auditory structures, and this has been suggested as a neural substrate for tinnitus. Using a guinea pig model we have previously demonstrated that for some time after cochlear trauma, central hyperactivity is dependent on peripheral afferent drive and only later becomes generated intrinsically within central structures. Furosemide, a loop diuretic, reduces spontaneous firing of auditory afferents. We investigated in our guinea pig model the efficacy of furosemide in reducing 1) spontaneous firing of auditory afferents, using the spectrum of neural noise (SNN) from round window recording, 2) hyperactivity in inferior colliculus, using extracellular single neuron recordings and 3) tinnitus at early time-points after cochlear trauma. Tinnitus was assessed using gap prepulse inhibition of acoustic startle (GPIAS). Intraperitoneal furosemide, but not saline, caused a marked decrease in both SNN and central hyperactivity. Intracochlear perfusion with furosemide similarly reversed central hyperactivity. In animals in which GPIAS measurements suggested the presence of tinnitus (reduced GPIAS), this could be reversed with an intraperitoneal injection with furosemide but not saline. The results are consistent with furosemide reducing central hyperactivity and behavioural signs of tinnitus by acting peripherally to decrease spontaneous firing of auditory afferents. The data support the notion that hyperactivity may be involved in the generation of tinnitus and further suggest that there may be a therapeutic window after cochlear trauma using drug treatments that target peripheral spontaneous activity.
Conflict of interest statement
Figures
Similar articles
-
Effects of pulsatile electrical stimulation of the round window on central hyperactivity after cochlear trauma in guinea pig.Hear Res. 2016 May;335:128-137. doi: 10.1016/j.heares.2016.03.001. Epub 2016 Mar 10. Hear Res. 2016. PMID: 26970475
-
Effects of chronic furosemide on central neural hyperactivity and cochlear thresholds after cochlear trauma in Guinea pig.Front Neurol. 2014 Aug 8;5:146. doi: 10.3389/fneur.2014.00146. eCollection 2014. Front Neurol. 2014. PMID: 25152746 Free PMC article.
-
Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity.Neuroscience. 2009 Dec 1;164(2):733-46. doi: 10.1016/j.neuroscience.2009.08.036. Epub 2009 Aug 20. Neuroscience. 2009. PMID: 19699277
-
Tinnitus: Maladaptive auditory-somatosensory plasticity.Hear Res. 2016 Apr;334:20-9. doi: 10.1016/j.heares.2015.06.005. Epub 2015 Jun 12. Hear Res. 2016. PMID: 26074307 Free PMC article. Review.
-
Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus.Hear Res. 2013 Jan;295:100-13. doi: 10.1016/j.heares.2012.11.016. Epub 2012 Nov 27. Hear Res. 2013. PMID: 23201030 Free PMC article. Review.
Cited by
-
Excitatory Repetitive Transcranial Magnetic Stimulation Over Prefrontal Cortex in a Guinea Pig Model Ameliorates Tinnitus.Front Neurosci. 2021 Jul 22;15:693935. doi: 10.3389/fnins.2021.693935. eCollection 2021. Front Neurosci. 2021. PMID: 34366777 Free PMC article.
-
Effects of long-term salicylate administration on synaptic ultrastructure and metabolic activity in the rat CNS.Sci Rep. 2016 Apr 12;6:24428. doi: 10.1038/srep24428. Sci Rep. 2016. PMID: 27068004 Free PMC article.
-
Use of the guinea pig in studies on the development and prevention of acquired sensorineural hearing loss, with an emphasis on noise.J Acoust Soc Am. 2019 Nov;146(5):3743. doi: 10.1121/1.5132711. J Acoust Soc Am. 2019. PMID: 31795705 Free PMC article. Review.
-
An Integrative Tinnitus Model Based on Sensory Precision.Trends Neurosci. 2016 Dec;39(12):799-812. doi: 10.1016/j.tins.2016.10.004. Epub 2016 Nov 18. Trends Neurosci. 2016. PMID: 27871729 Free PMC article. Review.
-
Long-term Administration of Salicylate-induced Changes in BDNF Expression and CREB Phosphorylation in the Auditory Cortex of Rats.Otol Neurotol. 2018 Mar;39(3):e173-e180. doi: 10.1097/MAO.0000000000001717. Otol Neurotol. 2018. PMID: 29342042 Free PMC article.
References
-
- Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. TRENDS in neuroscience 27: 676–682. - PubMed
-
- Shargorodsky J, Curhan GC, Farwell WR (2010) Prevalence and characteristics of tinnitus among US adults. Am J Med 123: 711–718. - PubMed
-
- Vio MM, Holme RH (2005) Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today 10: 1263–1265. - PubMed
-
- Axelsson A, Ringdahl A (1989) Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23: 53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

