Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial
- PMID: 24835842
- PMCID: PMC4233253
- DOI: 10.1016/S0140-6736(14)60162-8
Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial
Abstract
Background: Antiretroviral therapy reduces the risk of tuberculosis, but tuberculosis is more common in people with HIV than in people without HIV. We aimed to assess the effect of isoniazid preventive therapy on the risk of tuberculosis in people infected with HIV-1 concurrently receiving antiretroviral therapy.
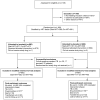
Methods: For this pragmatic randomised double-blind, placebo-controlled trial in Khayelitsha, South Africa, we randomly assigned (1:1) patients to receive either isoniazid preventive therapy or a placebo for 12 months (could be completed during 15 months). Randomisation was done with random number generator software. Participants, physicians, and pharmacy staff were masked to group assignment. The primary endpoint was time to development of incident tuberculosis (definite, probable, or possible). We excluded tuberculosis at screening by sputum culture. We did a modified intention-to-treat analysis and excluded all patients randomly assigned to groups who withdrew before receiving study drug or whose baseline sputum culture results suggested prevalent tuberculosis. This study is registered with ClinicalTrials.gov, number NCT00463086.
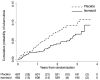
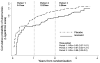
Findings: 1329 participants were randomly assigned to receive isoniazid preventive therapy (n=662) or placebo (n=667) between Jan 31, 2008, and Sept 31, 2011, and contributed 3227 person-years of follow-up to the analysis. We recorded 95 incident cases of tuberculosis; 37 were in the isoniazid preventive therapy group (2·3 per 100 person-years, 95% CI 1·6-3·1), and 58 in the placebo group (3·6 per 100 person-years, 2·8-4·7; hazard ratio [HR] 0·63, 95% CI 0·41-0·94). Study drug was discontinued because of grade 3 or 4 raised alanine transaminase concentrations in 19 of 662 individuals in the isoniazid preventive therapy group and ten of the 667 individuals in the placebo group (risk ratio 1·9, 95% CI 0·90-4·09). We noted no evidence that the effect of isoniazid preventive therapy was restricted to patients who were positive on tuberculin skin test or interferon gamma release assay (adjusted HR for patients with negative tests 0·43 [0·21-0·86] and 0·43 [0·20-0·96]; for positive tests 0·86 [0·37-2·00] and 0·55 [0·26-1·24], respectively).
Interpretation: Without a more predictive test or a multivariate algorithm that predicts benefit, isoniazid preventive therapy should be recommended to all patients receiving antiretroviral therapy in moderate or high incidence areas irrespective of tuberculin skin test or interferon gamma release assay status.
Funding: Department of Health of South Africa, the Wellcome Trust, Médecins Sans Frontières, European and Developing Countries Clinical Trials Partnership, Foundation for Innovation and New Diagnostics, the European Union, and Hasso Plattner (Institute of Infectious Diseases and Molecular Medicine, University of Cape Town).
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Preventive treatment for tuberculosis in people with HIV.Lancet. 2014 Aug 23;384(9944):644-6. doi: 10.1016/S0140-6736(14)60268-3. Epub 2014 May 13. Lancet. 2014. PMID: 24835843 No abstract available.
-
Isoniazid prevented active tuberculosis in patients with HIV treated with antiretroviral therapy.Ann Intern Med. 2014 Sep 16;161(6):JC12. doi: 10.7326/0003-4819-161-6-201409160-02012. Ann Intern Med. 2014. PMID: 25222412 No abstract available.
References
-
- WHO . WHO 2011 Report: Global Tuberculosis Control. World Health Organization; Geneva: [Access date: 15 September 2012]. 2011. URL: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

