Rosuvastatin for sepsis-associated acute respiratory distress syndrome
- PMID: 24835849
- PMCID: PMC4241052
- DOI: 10.1056/NEJMoa1401520
Rosuvastatin for sepsis-associated acute respiratory distress syndrome
Abstract
Background: In the acute respiratory distress syndrome (ARDS), inflammation in the lungs and other organs can cause life-threatening organ failure. Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) can modulate inflammatory responses. Previous observational studies suggested that statins improved clinical outcomes in patients with sepsis. We hypothesized that rosuvastatin therapy would improve clinical outcomes in critically ill patients with sepsis-associated ARDS.
Methods: We conducted a multicenter trial in which patients with sepsis-associated ARDS were randomly assigned to receive either enteral rosuvastatin or placebo in a double-blind manner. The primary outcome was mortality before hospital discharge home or until study day 60 if the patient was still in a health care facility. Secondary outcomes included the number of ventilator-free days (days that patients were alive and breathing spontaneously) to day 28 and organ-failure-free days to day 14.
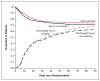
Results: The study was stopped because of futility after 745 of an estimated 1000 patients had been enrolled. There was no significant difference between study groups in 60-day in-hospital mortality (28.5% with rosuvastatin and 24.9% with placebo, P=0.21) or in mean (±SD) ventilator-free days (15.1±10.8 with rosuvastatin and 15.1±11.0 with placebo, P=0.96). The groups were well matched with respect to demographic and key physiological variables. Rosuvastatin therapy, as compared with placebo, was associated with fewer days free of renal failure to day 14 (10.1±5.3 vs. 11.0±4.7, P=0.01) and fewer days free of hepatic failure to day 14 (10.8±5.0 vs. 11.8±4.3, P=0.003). Rosuvastatin was not associated with an increased incidence of serum creatine kinase levels that were more than 10 times the upper limit of the normal range.
Conclusions: Rosuvastatin therapy did not improve clinical outcomes in patients with sepsis-associated ARDS and may have contributed to hepatic and renal organ dysfunction. (Funded by the National Heart, Lung, and Blood Institute and the Investigator-Sponsored Study Program of AstraZeneca; ClinicalTrials.gov number, NCT00979121.).
Figures
Comment in
-
Statin strikeout.N Engl J Med. 2014 Jun 5;370(23):2240-1. doi: 10.1056/NEJMe1405032. Epub 2014 May 18. N Engl J Med. 2014. PMID: 24835850 No abstract available.
-
Rosuvastatin for sepsis-associated ARDS.N Engl J Med. 2014 Sep 4;371(10):968-9. doi: 10.1056/NEJMc1408401. N Engl J Med. 2014. PMID: 25184876 No abstract available.
-
Rosuvastatin for sepsis-associated ARDS.N Engl J Med. 2014 Sep 4;371(10):968. doi: 10.1056/NEJMc1408401. N Engl J Med. 2014. PMID: 25184877 No abstract available.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. - PubMed
-
- Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–3. - PubMed
-
- Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–6. - PubMed
-
- Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
