The dynamics of SecM-induced translational stalling
- PMID: 24836001
- PMCID: PMC4059775
- DOI: 10.1016/j.celrep.2014.04.033
The dynamics of SecM-induced translational stalling
Abstract
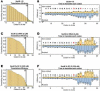
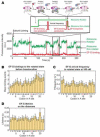
SecM is an E. coli secretion monitor capable of stalling translation on the prokaryotic ribosome without cofactors. Biochemical and structural studies have demonstrated that the SecM nascent chain interacts with the 50S subunit exit tunnel to inhibit peptide bond formation. However, the timescales and pathways of stalling on an mRNA remain undefined. To provide a dynamic mechanism for stalling, we directly tracked the dynamics of elongation on ribosomes translating the SecM stall sequence (FSTPVWISQAQGIRAGP) using single-molecule fluorescence techniques. Within 1 min, three peptide-ribosome interactions work cooperatively over the last five codons of the SecM sequence, leading to severely impaired elongation rates beginning from the terminal proline and lasting four codons. Our results suggest that stalling is tightly linked to the dynamics of elongation and underscore the roles that the exit tunnel and nascent chain play in controlling fundamental steps in translation.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Aitken CE, Petrov A, Puglisi JD. Single ribosome dynamics and the mechanism of translation. Annual review of biophysics. 2010;39:491–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

