Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial
- PMID: 24836205
- PMCID: PMC4154311
- DOI: 10.1016/S2213-2600(14)70100-6
Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial
Abstract
Background: Ataluren was developed to restore functional protein production in genetic disorders caused by nonsense mutations, which are the cause of cystic fibrosis in 10% of patients. This trial was designed to assess the efficacy and safety of ataluren in patients with nonsense-mutation cystic fibrosis.
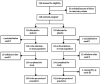
Methods: This randomised, double-blind, placebo-controlled, phase 3 study enrolled patients from 36 sites in 11 countries in North America and Europe. Eligible patients with nonsense-mutation cystic fibrosis (aged ≥ 6 years; abnormal nasal potential difference; sweat chloride >40 mmol/L; forced expiratory volume in 1 s [FEV1] ≥ 40% and ≤ 90%) were randomly assigned by interactive response technology to receive oral ataluren (10 mg/kg in morning, 10 mg/kg midday, and 20 mg/kg in evening) or matching placebo for 48 weeks. Randomisation used a block size of four, stratified by age, chronic inhaled antibiotic use, and percent-predicted FEV1. The primary endpoint was relative change in percent-predicted FEV1 from baseline to week 48, analysed in all patients with a post-baseline spirometry measurement. This study is registered with ClinicalTrials.gov, number NCT00803205.
Findings: Between Sept 8, 2009, and Nov 30, 2010, 238 patients were randomly assigned, of whom 116 in each treatment group had a valid post-baseline spirometry measurement. Relative change from baseline in percent-predicted FEV1 did not differ significantly between ataluren and placebo at week 48 (-2.5% vs -5.5%; difference 3.0% [95% CI -0.8 to 6.3]; p=0.12). The number of pulmonary exacerbations did not differ significantly between treatment groups (rate ratio 0.77 [95% CI 0.57-1.05]; p=0.0992). However, post-hoc analysis of the subgroup of patients not using chronic inhaled tobramycin showed a 5.7% difference (95% CI 1.5-10.1) in relative change from baseline in percent-predicted FEV1 between the ataluren and placebo groups at week 48 (-0.7% [-4.0 to 2.1] vs -6.4% [-9.8 to -3.7]; nominal p=0.0082), and fewer pulmonary exacerbations in the ataluern group (1.42 events [0.9-1.9] vs 2.18 events [1.6-2.7]; rate ratio 0.60 [0.42-0.86]; nominal p=0.0061). Safety profiles were generally similar for ataluren and placebo, except for the occurrence of increased creatinine concentrations (ie, acute kidney injury), which occurred in 18 (15%) of 118 patients in the ataluren group compared with one (<1%) of 120 patients in the placebo group. No life-threatening adverse events or deaths were reported in either group.
Interpretation: Although ataluren did not improve lung function in the overall population of nonsense-mutation cystic fibrosis patients who received this treatment, it might be beneficial for patients not taking chronic inhaled tobramycin.
Funding: PTC Therapeutics, Cystic Fibrosis Foundation, US Food and Drug Administration's Office of Orphan Products Development, and the National Institutes of Health.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Targeting nonsense-mediated cystic fibrosis: is it premature to stop now?Lancet Respir Med. 2014 Jul;2(7):509-11. doi: 10.1016/S2213-2600(14)70108-0. Epub 2014 May 15. Lancet Respir Med. 2014. PMID: 24836206 No abstract available.
-
Clinical Trials of Novel Treatments for Cystic Fibrosis.Am J Respir Crit Care Med. 2016 Mar 1;193(5):569-71. doi: 10.1164/rccm.201509-1734RR. Am J Respir Crit Care Med. 2016. PMID: 26765316 No abstract available.
References
-
- Boyle M, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. The Lancet Respiratory Med. 2013 Apr;1(2):158–163. - PubMed
-
- US Food and Drug Administration. Drug Approval Package. Kalydeco (ivacaftor 150 mg Tablets. FDA Web site. [Accessed February 06, 2013]; Updated January 31, 2012. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203188s000TOC.cfm.
-
- Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1993 Oct 28;329(18):1308–13. - PubMed
-
- Kerem E, Kerem B. Genotype-phenotype correlations in cystic fibrosis. PediatrPulmonol. 1996 Dec;22(6):387–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

