Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis
- PMID: 24836309
- PMCID: PMC4116664
- DOI: 10.1056/NEJMoa1401739
Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis
Abstract
Background: Acetylcysteine has been suggested as a beneficial treatment for idiopathic pulmonary fibrosis, although data from placebo-controlled studies are lacking.
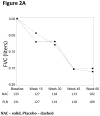
Methods: In our initial double-blind, placebo-controlled trial, we randomly assigned patients who had idiopathic pulmonary fibrosis with mild-to-moderate impairment in pulmonary function to receive a three-drug regimen of prednisone, azathioprine, and acetylcysteine; acetylcysteine alone; or placebo. The study was interrupted owing to safety concerns associated with the three-drug regimen. The trial continued as a two-group study (acetylcysteine vs. placebo) without other changes; 133 and 131 patients were enrolled in the acetylcysteine and placebo groups, respectively. The primary outcome was the change in forced vital capacity (FVC) over a 60-week period.
Results: At 60 weeks, there was no significant difference in the change in FVC between the acetylcysteine group and the placebo group (-0.18 liters and -0.19 liters, respectively; P=0.77). In addition, there were no significant differences between the acetylcysteine group and the placebo group in the rates of death (4.9% vs. 2.5%, P=0.30 by the log-rank test) or acute exacerbation (2.3% in each group, P>0.99).
Conclusions: As compared with placebo, acetylcysteine offered no significant benefit with respect to the preservation of FVC in patients with idiopathic pulmonary fibrosis with mild-to-moderate impairment in lung function. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov number, NCT00650091.).
Figures
References
-
- Peikert T, Daniels CE, Beebe TJ, Meyer KC, Ryu JH. Assessment of current practice in the diagnosis and therapy of idiopathic pulmonary fibrosis. Respir Med. 2008;102:1342–8. - PubMed
-
- Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. - PubMed
-
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL080413/HL/NHLBI NIH HHS/United States
- U10 HL080513/HL/NHLBI NIH HHS/United States
- U10 HL080543/HL/NHLBI NIH HHS/United States
- U10HL080510/HL/NHLBI NIH HHS/United States
- U10HL080370/HL/NHLBI NIH HHS/United States
- U10 HL080685/HL/NHLBI NIH HHS/United States
- U10HL080413/HL/NHLBI NIH HHS/United States
- U10HL080383/HL/NHLBI NIH HHS/United States
- U10HL080371/HL/NHLBI NIH HHS/United States
- U10 HL080274/HL/NHLBI NIH HHS/United States
- U10HL080513/HL/NHLBI NIH HHS/United States
- U10HL080685/HL/NHLBI NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- U10 HL080371/HL/NHLBI NIH HHS/United States
- U10HL080411/HL/NHLBI NIH HHS/United States
- U10 HL080383/HL/NHLBI NIH HHS/United States
- U10 HL080370/HL/NHLBI NIH HHS/United States
- U10HL080571/HL/NHLBI NIH HHS/United States
- U10 HL080411/HL/NHLBI NIH HHS/United States
- U10HL080509/HL/NHLBI NIH HHS/United States
- U10HL080274/HL/NHLBI NIH HHS/United States
- U10 HL080509/HL/NHLBI NIH HHS/United States
- U10 HL080571/HL/NHLBI NIH HHS/United States
- U10 HL080510/HL/NHLBI NIH HHS/United States
- U10HL080543/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials