Role for the nicotinic cholinergic system in movement disorders; therapeutic implications
- PMID: 24836728
- PMCID: PMC4149916
- DOI: 10.1016/j.pharmthera.2014.05.004
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications
Abstract
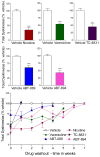
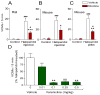
A large body of evidence using experimental animal models shows that the nicotinic cholinergic system is involved in the control of movement under physiological conditions. This work raised the question whether dysregulation of this system may contribute to motor dysfunction and whether drugs targeting nicotinic acetylcholine receptors (nAChRs) may be of therapeutic benefit in movement disorders. Accumulating preclinical studies now show that drugs acting at nAChRs improve drug-induced dyskinesias. The general nAChR agonist nicotine, as well as several nAChR agonists (varenicline, ABT-089 and ABT-894), reduces l-dopa-induced abnormal involuntary movements or dyskinesias up to 60% in parkinsonian nonhuman primates and rodents. These dyskinesias are potentially debilitating abnormal involuntary movements that arise as a complication of l-dopa therapy for Parkinson's disease. In addition, nicotine and varenicline decrease antipsychotic-induced abnormal involuntary movements in rodent models of tardive dyskinesia. Antipsychotic-induced dyskinesias frequently arise as a side effect of chronic drug treatment for schizophrenia, psychosis and other psychiatric disorders. Preclinical and clinical studies also show that the nAChR agonist varenicline improves balance and coordination in various ataxias. Lastly, nicotine has been reported to attenuate the dyskinetic symptoms of Tourette's disorder. Several nAChR subtypes appear to be involved in these beneficial effects of nicotine and nAChR drugs including α4β2*, α6β2* and α7 nAChRs (the asterisk indicates the possible presence of other subunits in the receptor). Overall, the above findings, coupled with nicotine's neuroprotective effects, suggest that nAChR drugs have potential for future drug development for movement disorders.
Keywords: Ataxia; Nicotine; Nicotinic acetylcholine receptors; Tardive dyskinesia; Tourette's syndrome; l-dopa-induced dyskinesias.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Potential Therapeutic Application for Nicotinic Receptor Drugs in Movement Disorders.Nicotine Tob Res. 2019 Feb 18;21(3):357-369. doi: 10.1093/ntr/nty063. Nicotine Tob Res. 2019. PMID: 30137517 Free PMC article. Review.
-
Evidence for a role for α6(∗) nAChRs in l-dopa-induced dyskinesias using Parkinsonian α6(∗) nAChR gain-of-function mice.Neuroscience. 2015 Jun 4;295:187-97. doi: 10.1016/j.neuroscience.2015.03.040. Epub 2015 Mar 24. Neuroscience. 2015. PMID: 25813704 Free PMC article.
-
Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice.Biochem Pharmacol. 2013 Oct 15;86(8):1153-62. doi: 10.1016/j.bcp.2013.06.027. Epub 2013 Jul 4. Biochem Pharmacol. 2013. PMID: 23831952 Free PMC article.
-
Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats.Neuropharmacology. 2011 May;60(6):861-8. doi: 10.1016/j.neuropharm.2010.12.032. Epub 2011 Jan 11. Neuropharmacology. 2011. PMID: 21232546 Free PMC article.
-
Nicotine and Nicotinic Receptor Drugs: Potential for Parkinson's Disease and Drug-Induced Movement Disorders.Int Rev Neurobiol. 2015;124:247-71. doi: 10.1016/bs.irn.2015.07.005. Epub 2015 Aug 18. Int Rev Neurobiol. 2015. PMID: 26472532 Review.
Cited by
-
Potential Therapeutic Application for Nicotinic Receptor Drugs in Movement Disorders.Nicotine Tob Res. 2019 Feb 18;21(3):357-369. doi: 10.1093/ntr/nty063. Nicotine Tob Res. 2019. PMID: 30137517 Free PMC article. Review.
-
Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias.Neurobiol Dis. 2016 Jul;91:47-58. doi: 10.1016/j.nbd.2016.02.019. Epub 2016 Feb 24. Neurobiol Dis. 2016. PMID: 26921469 Free PMC article.
-
Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson's disease.NPJ Parkinsons Dis. 2016 Feb 18;2:16001. doi: 10.1038/npjparkd.2016.1. eCollection 2016. NPJ Parkinsons Dis. 2016. PMID: 28725692 Free PMC article. Review.
-
Receptor Ligands as Helping Hands to L-DOPA in the Treatment of Parkinson's Disease.Biomolecules. 2019 Apr 9;9(4):142. doi: 10.3390/biom9040142. Biomolecules. 2019. PMID: 30970612 Free PMC article. Review.
-
Evidence for a role for α6(∗) nAChRs in l-dopa-induced dyskinesias using Parkinsonian α6(∗) nAChR gain-of-function mice.Neuroscience. 2015 Jun 4;295:187-97. doi: 10.1016/j.neuroscience.2015.03.040. Epub 2015 Mar 24. Neuroscience. 2015. PMID: 25813704 Free PMC article.
References
-
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. - PubMed
-
- Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006;30:1223–1233. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. - PubMed
-
- Anderson DJ, Malysz J, Gronlien JH, El Kouhen R, Hakerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M. Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem Pharmacol. 2009;78:844–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

