Dynamic interactions between cancer cells and the embryonic microenvironment regulate cell invasion and reveal EphB6 as a metastasis suppressor
- PMID: 24836890
- PMCID: PMC4498260
- DOI: 10.1158/1541-7786.MCR-13-0673
Dynamic interactions between cancer cells and the embryonic microenvironment regulate cell invasion and reveal EphB6 as a metastasis suppressor
Abstract
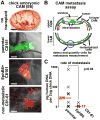
Metastatic dissemination drives the high mortality associated with melanoma. However, difficulties in visualizing in vivo cell dynamics during metastatic invasion have limited our understanding of these cell behaviors. Recent evidence has revealed that melanoma cells exploit portions of their ancestral embryonic neural crest emigration program to facilitate invasion. What remains to be determined is how embryonic microenvironmental signals influence invasive melanoma cell behavior, and whether these signals are relevant to human disease. To address these questions, we interrogated the role of the neural crest microenvironment in dictating the spatiotemporal pattern of melanoma cell invasion in the chick embryo using 2-photon time-lapse microscopy. Results reveal that both permissive and inhibitory neural crest microenvironmental signals regulate the timing and direction of melanoma invasion to coincide with the neural crest migration pattern. These cues include bidirectional signaling mediated through the ephrin family of receptor tyrosine kinases. We demonstrate that EphB6 reexpression forces metastatic melanoma cells to deviate from the canonical migration pattern observed in the chick embryo transplant model. Furthermore, EphB6-expressing melanoma cells display significantly reduced metastatic potential in a chorioallantoic membrane (CAM) metastasis assay. These data on melanoma invasion in the embryonic neural crest and CAM microenvironments identify EphB6 as a metastasis suppressor in melanoma, likely acting at the stage of intravasation.
Implications: This article links cellular metastasis to behaviors observed in the ancestrally related embryonic neural crest and demonstrates the powerful influence of the embryonic microenvironment in regulating cell migratory behavior.
©2014 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest: The authors report no conflict of interest
Figures






References
-
- Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–49. - PubMed
-
- Le Douarin NM, Kalcheim C. The Neural Crest. 2. Cambridge: Cambridge University Press; 1999.
-
- Trainor PA, Krumlauf R. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci. 2000;1:116–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

