Clinical and virological efficacy of etravirine plus two active Nucleos(t)ide analogs in an heterogeneous HIV-infected population
- PMID: 24836963
- PMCID: PMC4023987
- DOI: 10.1371/journal.pone.0097262
Clinical and virological efficacy of etravirine plus two active Nucleos(t)ide analogs in an heterogeneous HIV-infected population
Abstract
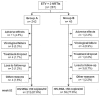
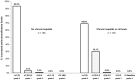
Etravirine (ETV) is recommended in combination with a boosted protease inhibitor plus an optimized background regimen for salvage therapy, but there is limited experience with its use in combination with two nucleos(t)ide reverse-transcriptase inhibitors (NRTIs). This multicenter study aimed to assess the efficacy of this combination in two scenarios: group A) subjects without virologic failure on or no experience with non-nucleoside reverse-transcriptase inhibitors (NNRTIs) switched due to adverse events and group B) subjects switched after a virologic failure on an efavirenz- or nevirapine-based regimen. The primary endpoint was efficacy at 52 weeks analysed by intention-to-treat. Virologic failure was defined as the inability to suppress plasma HIV-RNA to <50 copies/mL after 24 weeks on treatment, or a confirmed viral load >200 copies/mL in patients who had previously achieved a viral suppression or had an undetectable viral load at inclusion. Two hundred eighty seven patients were included. Treatment efficacy rates in group A and B were 88.0% (CI95, 83.9-92.1%) and 77.4% (CI95, 65.0-89.7%), respectively; the rates reached 97.2% (CI95, 95.1-99.3%) and 90.5% (CI95, 81.7-99.3), by on-treatment analysis. The once-a-day ETV treatment was as effective as the twice daily dosing regimen. Grade 1-2 adverse events were observed motivating a treatment switch in 4.2% of the subjects. In conclusion, ETV (once- or twice daily) plus two analogs is a suitable, well-tolerated combination both as a switching strategy and after failure with first generation NNRTIs, ensuring full drug activity.
Trial registration: ClinicalTrials.gov NCT01437241.
Conflict of interest statement
Figures

Similar articles
-
Etravirine: a review of its use in the management of treatment-experienced patients with HIV-1 infection.Drugs. 2012 Apr 16;72(6):847-69. doi: 10.2165/11209110-000000000-00000. Drugs. 2012. PMID: 22512366 Review.
-
Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results.AIDS. 2011 Nov 28;25(18):2249-58. doi: 10.1097/QAD.0b013e32834c4c06. AIDS. 2011. PMID: 21881478 Clinical Trial.
-
Etravirine and rilpivirine: nonnucleoside reverse transcriptase inhibitors with activity against human immunodeficiency virus type 1 strains resistant to previous nonnucleoside agents.Pharmacotherapy. 2009 Mar;29(3):281-94. doi: 10.1592/phco.29.3.281. Pharmacotherapy. 2009. PMID: 19249947 Review.
-
Dual therapy combining raltegravir with etravirine maintains a high level of viral suppression over 96 weeks in long-term experienced HIV-infected individuals over 45 years on a PI-based regimen: results from the Phase II ANRS 163 ETRAL study.J Antimicrob Chemother. 2019 Sep 1;74(9):2742-2751. doi: 10.1093/jac/dkz224. J Antimicrob Chemother. 2019. PMID: 31269208
-
Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial.Lancet Infect Dis. 2015 Jul;15(7):785-92. doi: 10.1016/S1473-3099(15)00096-1. Epub 2015 Jun 7. Lancet Infect Dis. 2015. PMID: 26062880 Clinical Trial.
Cited by
-
Etravirine combined with antiretrovirals other than darunavir/ritonavir for HIV-1-infected, treatment-experienced adults: Week 48 results of a phase IV trial.SAGE Open Med. 2017 Jan 18;5:2050312116686482. doi: 10.1177/2050312116686482. eCollection 2017. SAGE Open Med. 2017. PMID: 28382208 Free PMC article.
-
Therapy-Emergent Drug Resistance to Integrase Strand Transfer Inhibitors in HIV-1 Patients: A Subgroup Meta-Analysis of Clinical Trials.PLoS One. 2016 Aug 17;11(8):e0160087. doi: 10.1371/journal.pone.0160087. eCollection 2016. PLoS One. 2016. PMID: 27532886 Free PMC article.
References
-
- Andries K, Azijn H, Thielemans T, Ludovici D, Kukla M, et al. (2004) TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 48: 4680–4686. - PMC - PubMed
-
- Gazzard BG, Pozniak AL, Rosenbaum W, Yeni GP, Staszewski S, et al. (2003) An open-label assessment of TMC 125 - a new, next-generation NNRTI, for 7 days in HIV-1 infected individuals with NNRTI resistance. AIDS. 17: F49–54. - PubMed
-
- Gruzdev B, Rakhmanova A, Doubovskaya E, Yakovlev A, Peeters M, et al. (2009) A randomized, double-blind, placebo-controlled trial of TMC125 as 7-day monotherapy in antiretroviral naive, HIV-1 infected subjects. AIDS (17) 2487–2494. - PubMed
-
- Katlama C, Haubrich R, Lalezari J, Lazzarin A, Madruga JV, et al. (2009) Efficacy and safety of Etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 23: 2289–2300. - PubMed
-
- Katlama C, Clotet B, Mills A, Trottier B, Molina JM, et al. (2010) Efficacy and safety of Etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther. 15: 1045–1052. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

