Biosynthesis of wyosine derivatives in tRNA(Phe) of Archaea: role of a remarkable bifunctional tRNA(Phe):m1G/imG2 methyltransferase
- PMID: 24837075
- PMCID: PMC4024628
- DOI: 10.1261/rna.043315.113
Biosynthesis of wyosine derivatives in tRNA(Phe) of Archaea: role of a remarkable bifunctional tRNA(Phe):m1G/imG2 methyltransferase
Abstract
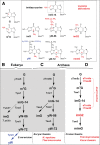
The presence of tricyclic wyosine derivatives 3'-adjacent to anticodon is a hallmark of tRNA(Phe) in eukaryotes and archaea. In yeast, formation of wybutosine (yW) results from five enzymes acting in a strict sequential order. In archaea, the intermediate compound imG-14 (4-demethylwyosine) is a target of three different enzymes, leading to the formation of distinct wyosine derivatives (yW-86, imG, and imG2). We focus here on a peculiar methyltransferase (aTrm5a) that catalyzes two distinct reactions: N(1)-methylation of guanosine and C(7)-methylation of imG-14, whose function is to allow the production of isowyosine (imG2), an intermediate of the 7-methylwyosine (mimG) biosynthetic pathway. Based on the formation of mesomeric forms of imG-14, a rationale for such dual enzymatic activities is proposed. This bifunctional tRNA:m(1)G/imG2 methyltransferase, acting on two chemically distinct guanosine derivatives located at the same position of tRNA(Phe), is unique to certain archaea and has no homologs in eukaryotes. This enzyme here referred to as Taw22, probably played an important role in the emergence of the multistep biosynthetic pathway of wyosine derivatives in archaea and eukaryotes.
Keywords: COG2520; archaea; methyltransferase; modification; transfer RNA.
© 2014 Urbonavičius et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures

References
-
- Bujnicki JM 2000. Phylogenomic analysis of 16S rRNA:(guanine-N2) methyltransferases suggests new family members and reveals highly conserved motifs and a domain structure similar to other nucleic acid amino-methyltransferases. FASEB J 14: 2365–2368 - PubMed
-
- Cannon LM, Butler FN, Wan W, Zhou ZS 2002. A stereospecific colorimetric assay for (S,S)-adenosylmethionine quantification based on thiopurine methyltransferase-catalyzed thiol methylation. Anal Biochem 308: 358–363 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
