The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions
- PMID: 24837393
- PMCID: PMC4148806
- DOI: 10.1128/AEM.01048-14
The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions
Abstract
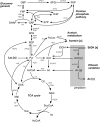
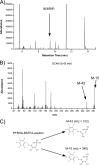
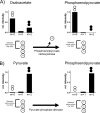
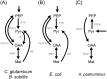
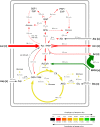
Acetic acid bacteria (AAB) play an important role during cocoa fermentation, as their main product, acetate, is a major driver for the development of the desired cocoa flavors. Here, we investigated the specialized metabolism of these bacteria under cocoa pulp fermentation-simulating conditions. A carefully designed combination of parallel 13C isotope labeling experiments allowed the elucidation of intracellular fluxes in the complex environment of cocoa pulp, when lactate and ethanol were included as primary substrates among undefined ingredients. We demonstrate that AAB exhibit a functionally separated metabolism during coconsumption of two-carbon and three-carbon substrates. Acetate is almost exclusively derived from ethanol, while lactate serves for the formation of acetoin and biomass building blocks. Although this is suboptimal for cellular energetics, this allows maximized growth and conversion rates. The functional separation results from a lack of phosphoenolpyruvate carboxykinase and malic enzymes, typically present in bacteria to interconnect metabolism. In fact, gluconeogenesis is driven by pyruvate phosphate dikinase. Consequently, a balanced ratio of lactate and ethanol is important for the optimum performance of AAB. As lactate and ethanol are individually supplied by lactic acid bacteria and yeasts during the initial phase of cocoa fermentation, respectively, this underlines the importance of a well-balanced microbial consortium for a successful fermentation process. Indeed, AAB performed the best and produced the largest amounts of acetate in mixed culture experiments when lactic acid bacteria and yeasts were both present.
Figures



References
-
- Biehl B, Passern D, Sagemann W. 1982. Effect of acetic acid on subcellular structures of cocoa bean cotyledons. J. Sci. Food Agric. 33:1101–1109. 10.1002/jsfa.2740331107 - DOI
-
- Quesnel VC. 1965. Agents inducing the death of cacao seeds during fermentation. J. Sci. Food Agric. 16:441–447. 10.1002/jsfa.2740160804 - DOI
-
- Forsyth WGC, Quesnel VC. 1963. The mechanism of cacao curing. Adv. Enzymol. Relat. Areas Mol. Biol. 25:457–492 - PubMed
-
- Camu N, de Winter T, Addo SK, Takrama JS, Bernaert H, de Vuyst L. 2008. Fermentation of cocoa beans: influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 88:2288–2297. 10.1002/jsfa.3349 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

