PDE5 inhibitor efficacy is estrogen dependent in female heart disease
- PMID: 24837433
- PMCID: PMC4089449
- DOI: 10.1172/JCI70731
PDE5 inhibitor efficacy is estrogen dependent in female heart disease
Abstract
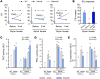
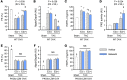
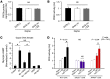
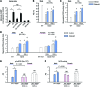
Inhibition of cGMP-specific phosphodiesterase 5 (PDE5) ameliorates pathological cardiac remodeling and has been gaining attention as a potential therapy for heart failure. Despite promising results in males, the efficacy of the PDE5 inhibitor sildenafil in female cardiac pathologies has not been determined and might be affected by estrogen levels, given the hormone's involvement in cGMP synthesis. Here, we determined that the heart-protective effect of sildenafil in female mice depends on the presence of estrogen via a mechanism that involves myocyte eNOS-dependent cGMP synthesis and the cGMP-dependent protein kinase Iα (PKGIα). Sildenafil treatment failed to exert antiremodeling properties in female pathological hearts from Gαq-overexpressing or pressure-overloaded mice after ovary removal; however, estrogen replacement restored the effectiveness of sildenafil in these animals. In females, sildenafil-elicited myocardial PKG activity required estrogen, which stimulated tonic cardiomyocyte cGMP synthesis via an eNOS/soluble guanylate cyclase pathway. In contrast, eNOS activation, cGMP synthesis, and sildenafil efficacy were not estrogen dependent in male hearts. Estrogen and sildenafil had no impact on pressure-overloaded hearts from animals expressing dysfunctional PKGIα, indicating that PKGIα mediates antiremodeling effects. These results support the importance of sex differences in the use of PDE5 inhibitors for treating heart disease and the critical role of estrogen status when these agents are used in females.
Figures
Comment in
-
Sex, drugs, and trial design: sex influences the heart and drug responses.J Clin Invest. 2014 Jun;124(6):2375-7. doi: 10.1172/JCI76262. Epub 2014 May 16. J Clin Invest. 2014. PMID: 24837428 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

