Challenges in vaccination of neonates, infants and young children
- PMID: 24837502
- PMCID: PMC4135535
- DOI: 10.1016/j.vaccine.2014.05.008
Challenges in vaccination of neonates, infants and young children
Abstract
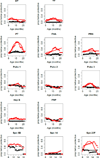
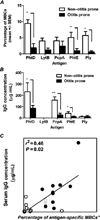
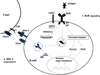
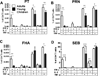
All neonates, infants and young children receive multiple priming doses and booster vaccinations in the 1st and 2nd year of life to prevent infections by viral and bacterial pathogens. Despite high vaccine compliance, outbreaks of vaccine-preventable infections are occurring worldwide. These data strongly argue for an improved understanding of the immune responses of neonates, infants and young children to vaccine antigens and further study of the exploitable mechanisms to achieve more robust and prolonged immunity with fewer primary and booster vaccinations in the pediatric population. This review will focus on our recent work involving infant and young child immunity following routine recommended vaccinations. The discussion will address vaccine responses with respect to four areas: (1) systemic antibody responses, (2) memory B-cell generation, (3) CD4 T-cell responses, and (4) APC function.
Keywords: Antigen presenting cells; B cells; B-cell receptor; CD4 T-cells; Children; Dendritic cells; Haemophilus influenzae; Infants; MHC II; Neonate; Streptococcus pneumoniae; T cells; T-cell receptor; Toll-like receptor; Vaccination; cytokines; immunologic memory; pediatric vaccines.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Casey JR, Pichichero ME. Acellular pertussis vaccine safety and efficacy in children, adolescents and adults. Drugs. 2005;65:1367–1389. - PubMed
-
- Fay KE, Lai J, Bocchini JA., Jr Update on childhood and adolescent immunizations: selected review of US recommendations and literature: part 1. CurrOpinPediatr. 2011;23:460–469. - PubMed
-
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, et al. Challenges in infant immunity: implications for responses to infection and vaccines. NatImmunol. 2011;12:189–194. - PubMed
-
- Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29:8215–8221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

