Argonaute-2 promotes miR-18a entry in human brain endothelial cells
- PMID: 24837588
- PMCID: PMC4309089
- DOI: 10.1161/JAHA.114.000968
Argonaute-2 promotes miR-18a entry in human brain endothelial cells
Abstract
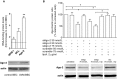
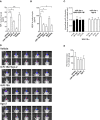
Background: Cerebral arteriovenous malformation (AVM) is a vascular disease exhibiting abnormal blood vessel morphology and function. miR-18a ameliorates the abnormal characteristics of AVM-derived brain endothelial cells (AVM-BEC) without the use of transfection reagents. Hence, our aim was to identify the mechanisms by which miR-18a is internalized by AVM-BEC. Since AVM-BEC overexpress RNA-binding protein Argonaute-2 (Ago-2) we explored the clinical potential of Ago-2 as a systemic miRNA carrier.
Methods and results: Primary cultures of AVM-BEC were isolated from surgical specimens and tested for endogenous miR-18a levels using qPCR. Conditioned media (CM) was derived from AVM-BEC cultures (AVM-BEC-CM). AVM-BEC-CM significantly enhanced miR-18a internalization. Ago-2 was detected using western blotting and immunostaining techniques. Ago-2 was highly expressed in AVM-BEC; and siAgo-2 decreased miR-18a entry into brain-derived endothelial cells. Only brain-derived endothelial cells were responsive to the Ago-2/miR-18a complex and not other cell types tested. Secreted products (eg, thrombospondin-1 [TSP-1]) were tested using ELISA. Brain endothelial cells treated with the Ago-2/miR-18a complex in vitro increased TSP-1 secretion. In the in vivo angiogenesis glioma model, animals were treated with miR-18a in combination with Ago-2. Plasma was obtained and tested for TSP-1 and vascular endothelial growth factor (VEGF)-A. In this angiogenesis model, the Ago-2/miR-18a complex caused a significant increase in TSP-1 and decrease in VEGF-A secretion in the plasma.
Conclusions: Ago-2 facilitates miR-18a entry into brain endothelial cells in vitro and in vivo. This study highlights the clinical potential of Ago-2 as a miRNA delivery platform for the treatment of brain vascular diseases.
Keywords: Argonaute‐2; angiogenesis; arteriovenous malformation; brain endothelial cells; miR‐18a.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures




Similar articles
-
miR-18a Inhibits BMP4 and HIF-1α Normalizing Brain Arteriovenous Malformations.Circ Res. 2020 Oct 9;127(9):e210-e231. doi: 10.1161/CIRCRESAHA.119.316317. Epub 2020 Aug 5. Circ Res. 2020. PMID: 32755283
-
MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function.Stroke. 2014 Jan;45(1):293-7. doi: 10.1161/STROKEAHA.113.003578. Epub 2013 Nov 7. Stroke. 2014. PMID: 24203843
-
Thrombospondin-1 modulates the angiogenic phenotype of human cerebral arteriovenous malformation endothelial cells.Neurosurgery. 2011 May;68(5):1342-53; discussion 1353. doi: 10.1227/NEU.0b013e31820c0a68. Neurosurgery. 2011. PMID: 21307796
-
Deciphering the vascular labyrinth: role of microRNAs and candidate gene SNPs in brain AVM development - literature review.Neurol Res. 2020 Dec;42(12):1043-1054. doi: 10.1080/01616412.2020.1796380. Epub 2020 Jul 28. Neurol Res. 2020. PMID: 32723034 Review.
-
Molecular and cellular biology of cerebral arteriovenous malformations: a review of current concepts and future trends in treatment.Neurosurg Focus. 2014 Sep;37(3):E1. doi: 10.3171/2014.7.FOCUS14214. Neurosurg Focus. 2014. PMID: 25175428 Review.
Cited by
-
Nrp-1 Mediated Plasmatic Ago2 Binding miR-21a-3p Internalization: A Novel Mechanism for miR-21a-3p Accumulation in Renal Tubular Epithelial Cells during Sepsis.Biomed Res Int. 2020 Aug 18;2020:2370253. doi: 10.1155/2020/2370253. eCollection 2020. Biomed Res Int. 2020. PMID: 32923478 Free PMC article.
-
MicroRNA-18a-5p Administration Suppresses Retinal Neovascularization by Targeting FGF1 and HIF1A.Front Pharmacol. 2020 Mar 10;11:276. doi: 10.3389/fphar.2020.00276. eCollection 2020. Front Pharmacol. 2020. PMID: 32210827 Free PMC article.
-
Widespread tissue delivery of antagomiRs via intramuscular administration.Mol Ther Methods Clin Dev. 2025 May 19;33(2):101488. doi: 10.1016/j.omtm.2025.101488. eCollection 2025 Jun 12. Mol Ther Methods Clin Dev. 2025. PMID: 40519322 Free PMC article.
-
Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy.Antioxidants (Basel). 2020 Aug 4;9(8):705. doi: 10.3390/antiox9080705. Antioxidants (Basel). 2020. PMID: 32759750 Free PMC article. Review.
-
Argonaute-2 protects the neurovascular unit from damage caused by systemic inflammation.J Neuroinflammation. 2022 Jan 6;19(1):11. doi: 10.1186/s12974-021-02324-7. J Neuroinflammation. 2022. PMID: 34991639 Free PMC article.
References
-
- Yashar P, Amar AP, Giannotta SL, Yu C, Pagnini PG, Liu CY, Apuzzo ML. Cerebral arteriovenous malformations: issues of the interplay between stereotactic radiosurgery and endovascular surgical therapy. World Neurosurg. 2011; 75:638-647. - PubMed
-
- Haw CS, terBrugge K, Willinsky R, Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006; 104:226-232. - PubMed
-
- Jabbour MN, Elder JB, Samuelson CG, Khashabi S, Hofman FM, Giannotta SL, Liu CY. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery. 2009; 64:139-146. - PubMed
-
- Stapleton CJ, Armstrong DL, Zidovetzki R, Liu CY, Giannotta SL, Hofman FM. Thrombospondin‐1 modulates the angiogenic phenotype of human cerebral arteriovenous malformation endothelial cells. Neurosurgery. 2011; 68:1342-1353. - PubMed
-
- Ferreira R, Santos T, Amar A, Tahara SM, Chen TC, Giannotta SL, Hofman FM. Microrna‐18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke. 2014; 45:293-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

