Vatalanib population pharmacokinetics in patients with myelodysplastic syndrome: CALGB 10105 (Alliance)
- PMID: 24838014
- PMCID: PMC4243874
- DOI: 10.1111/bcp.12427
Vatalanib population pharmacokinetics in patients with myelodysplastic syndrome: CALGB 10105 (Alliance)
Abstract
Aims: Vatalanib is an oral anti-angiogenesis agent that inhibits vascular endothelial growth factor receptor tyrosine kinases, which in patients showed auto induction of metabolism and variability in pharmacokinetic (PK) disposition. The objective was to characterize the population PK and time-dependent change in vatalanib clearance and assess exposure-toxicity relationship in patients with myelodysplastic syndrome (MDS).
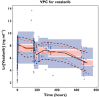
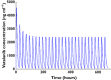
Methods: This was an open-label phase II study of vatalanib in MDS patients receiving 750-1250 mg once daily in 28-day cycles. Serial blood samples were obtained and plasma vatalanib concentrations measured by HPLC. Population PK analysis was performed using nonmem 7.2 with FO estimation since FOCE failed. The final model was evaluated using goodness-of-fit plots, bootstrap analysis, and visual predictive check.
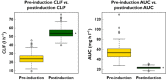
Results: Pharmacokinetic data were complete for 137 patients (86 M, 51 F), of median age 70 years (range 20-91). A one-compartment model with lagged first-order absorption and time-dependent change in oral clearance was fitted to the vatalanib plasma concentration versus time data. The population means for pre-induction and post-induction oral clearance were 24.1 l h(-1) (range: 9.6-45.5) and 54.9 l h(-1) (range: 39.8-75.6), respectively. The apparent oral clearance increased 2.3-fold, (range: 1.7-4.1-fold) from first dose to steady state. Our data did not identify a significant relationship of the predefined covariates with vatalanib pharmacokinetics, although power to detect such a relationship was limited.
Conclusions: Vatalanib pharmacokinetics were highly variable and the extent of auto induction was not determined to correlate with any of the pre-defined covariates.
Keywords: autoinduction; population pharmacokinetic model; time-dependent clearance; vatalanib.
© 2014 The British Pharmacological Society.
Figures
References
-
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. - PubMed
-
- Bussolino F, Albini A, Camussi G, Presta M, Viglietto G, Ziche M, Persico G. Role of soluble mediators in angiogenesis. Eur J Cancer. 1996;32A:2401–2412. - PubMed
-
- Padró T, Ruiz S, Bieker R, Bürger H, Steins M, Kienast J, Buchner T, Berdel WE, Mesters RM. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood. 2000;95:2637–2644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

