Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades
- PMID: 24838141
- PMCID: PMC4109754
- DOI: 10.1194/jlr.R049437
Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades
Abstract
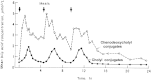
During the last 80 years there have been extraordinary advances in our knowledge of the chemistry and biology of bile acids. We present here a brief history of the major achievements as we perceive them. Bernal, a physicist, determined the X-ray structure of cholesterol crystals, and his data together with the vast chemical studies of Wieland and Windaus enabled the correct structure of the steroid nucleus to be deduced. Today, C24 and C27 bile acids together with C27 bile alcohols constitute most of the bile acid "family". Patterns of bile acid hydroxylation and conjugation are summarized. Bile acid measurement encompasses the techniques of GC, HPLC, and MS, as well as enzymatic, bioluminescent, and competitive binding methods. The enterohepatic circulation of bile acids results from vectorial transport of bile acids by the ileal enterocyte and hepatocyte; the key transporters have been cloned. Bile acids are amphipathic, self-associate in solution, and form mixed micelles with polar lipids, phosphatidylcholine in bile, and fatty acids in intestinal content during triglyceride digestion. The rise and decline of dissolution of cholesterol gallstones by the ingestion of 3,7-dihydroxy bile acids is chronicled. Scientists from throughout the world have contributed to these achievements.
Keywords: bile acid analysis; bile acid metabolism; bile acid physical chemistry; bile acid transport; enterohepatic circulation.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Thannhauser S. J., Lenke M. 1928. Ueber die Herkunft der Gallensäuren, Cholesterin-Gallensäurenbilanzen beim Hund mit totaler Gallenfistel. Arch. Exp. Pathol. Pharmakol. 1130: 292–307.
-
- Neubauer E. 1924. Über die Cholagoge Wirkung der Dehydrocholsäure beim Menschen. Klin. Wochenschr. 3: 883.
-
- Shimizu T. 1935. Über die Chemie and Physiologie der Gallensäuren. Verlag von M. Muramoto, Japan.
-
- Sarett L. H. 1946. Partial synthesis of pregnene 4 triol 17(β),20(β), 21 dione 3,11 and pregnene 4 diol 17(β), 21 trione 3,11,20 monoacetate. J. Biol. Chem. 162: 601–631. - PubMed
-
- Hillier S. G. 2007. Diamonds are forever: the cortisone legacy. J. Endocrinol. 195: 1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

