Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms
- PMID: 24838145
- PMCID: PMC4686340
- DOI: 10.1007/s12026-014-8535-y
Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms
Abstract
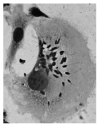
Leishmania amazonensis is an intracellular protozoan parasite responsible for chronic cutaneous leishmaniasis (CL). CL is a neglected tropical disease responsible for infecting millions of people worldwide. L. amazonensis promotes alteration of various signaling pathways that are essential for host cell survival. Specifically, through parasite-mediated phosphorylation of extracellular signal regulated kinase (ERK), L. amazonensis inhibits cell-mediated parasite killing and promotes its own survival by co-opting multiple host cell functions. In this review, we highlight Leishmania-host cell signaling alterations focusing on those specific to (1) motor proteins, (2) prevention of NADPH subunit phosphorylation impairing reactive oxygen species production, and (3) localized endosomal signaling to up-regulate ERK phosphorylation. This review will focus upon mechanisms and possible explanations as to how Leishmania spp. evades the various layers of defense employed by the host immune response.
Figures



Similar articles
-
Targeted extracellular signal-regulated kinase activation mediated by Leishmania amazonensis requires MP1 scaffold.Microbes Infect. 2014 Apr;16(4):328-36. doi: 10.1016/j.micinf.2013.12.006. Epub 2014 Jan 22. Microbes Infect. 2014. PMID: 24463270 Free PMC article.
-
In Vivo Infection with Leishmania amazonensis to Evaluate Parasite Virulence in Mice.J Vis Exp. 2020 Feb 20;(156). doi: 10.3791/60617. J Vis Exp. 2020. PMID: 32150165
-
MAPK/ERK activation in macrophages promotes Leishmania internalization and pathogenesis.Microbes Infect. 2024 Jul-Aug;26(5-6):105353. doi: 10.1016/j.micinf.2024.105353. Epub 2024 May 17. Microbes Infect. 2024. PMID: 38763478
-
Immunoregulation in human American leishmaniasis: balancing pathology and protection.Parasite Immunol. 2014 Aug;36(8):367-76. doi: 10.1111/pim.12100. Parasite Immunol. 2014. PMID: 24471648 Free PMC article. Review.
-
The Impact of Neutrophil Recruitment to the Skin on the Pathology Induced by Leishmania Infection.Front Immunol. 2021 Mar 1;12:649348. doi: 10.3389/fimmu.2021.649348. eCollection 2021. Front Immunol. 2021. PMID: 33732265 Free PMC article. Review.
Cited by
-
Leishmania amazonensis Subverts the Transcription Factor Landscape in Dendritic Cells to Avoid Inflammasome Activation and Stall Maturation.Front Immunol. 2020 Jun 9;11:1098. doi: 10.3389/fimmu.2020.01098. eCollection 2020. Front Immunol. 2020. PMID: 32582184 Free PMC article.
-
Py-CoMFA, docking, and molecular dynamics simulations of Leishmania (L.) amazonensis arginase inhibitors.Sci Rep. 2024 May 21;14(1):11575. doi: 10.1038/s41598-024-62520-2. Sci Rep. 2024. PMID: 38773273 Free PMC article.
-
Extracellular Vesicles Released by Leishmania (Leishmania) amazonensis Promote Disease Progression and Induce the Production of Different Cytokines in Macrophages and B-1 Cells.Front Microbiol. 2018 Dec 21;9:3056. doi: 10.3389/fmicb.2018.03056. eCollection 2018. Front Microbiol. 2018. PMID: 30627118 Free PMC article.
-
Leishmania Infection Engages Non-Receptor Protein Kinases Differentially to Persist in Infected Hosts.Front Immunol. 2016 Apr 18;7:146. doi: 10.3389/fimmu.2016.00146. eCollection 2016. Front Immunol. 2016. PMID: 27148265 Free PMC article. Review.
-
Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis.Molecules. 2014 Dec 15;19(12):21044-65. doi: 10.3390/molecules191221044. Molecules. 2014. PMID: 25517343 Free PMC article.
References
-
- Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. The American journal of tropical medicine and hygiene. 1995;53(3):256–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

