A genome-wide RNAi screen identifies potential drug targets in a C. elegans model of α1-antitrypsin deficiency
- PMID: 24838285
- PMCID: PMC4159156
- DOI: 10.1093/hmg/ddu236
A genome-wide RNAi screen identifies potential drug targets in a C. elegans model of α1-antitrypsin deficiency
Abstract
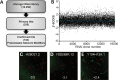
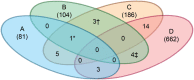
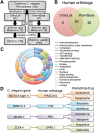
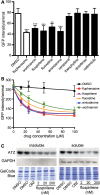
α1-Antitrypsin deficiency (ATD) is a common genetic disorder that can lead to end-stage liver and lung disease. Although liver transplantation remains the only therapy currently available, manipulation of the proteostasis network (PN) by small molecule therapeutics offers great promise. To accelerate the drug-discovery process for this disease, we first developed a semi-automated high-throughput/content-genome-wide RNAi screen to identify PN modifiers affecting the accumulation of the α1-antitrypsin Z mutant (ATZ) in a Caenorhabditis elegans model of ATD. We identified 104 PN modifiers, and these genes were used in a computational strategy to identify human ortholog-ligand pairs. Based on rigorous selection criteria, we identified four FDA-approved drugs directed against four different PN targets that decreased the accumulation of ATZ in C. elegans. We also tested one of the compounds in a mammalian cell line with similar results. This methodology also proved useful in confirming drug targets in vivo, and predicting the success of combination therapy. We propose that small animal models of genetic disorders combined with genome-wide RNAi screening and computational methods can be used to rapidly, economically and strategically prime the preclinical discovery pipeline for rare and neglected diseases with limited therapeutic options.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Huber R., Carrell R.W. Implications of the three-dimensional structure of a 1- antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–8966. - PubMed
-
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am. Rev. Respir. Dis. 1985;132:417–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

