A C. elegans model of human α1-antitrypsin deficiency links components of the RNAi pathway to misfolded protein turnover
- PMID: 24838286
- PMCID: PMC4159155
- DOI: 10.1093/hmg/ddu235
A C. elegans model of human α1-antitrypsin deficiency links components of the RNAi pathway to misfolded protein turnover
Abstract
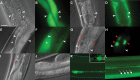
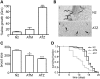
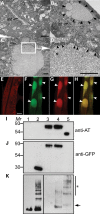
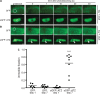
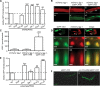
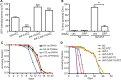
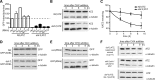
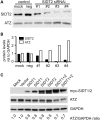
The accumulation of serpin oligomers and polymers within the endoplasmic reticulum (ER) causes cellular injury in patients with the classical form α1-antitrypsin deficiency (ATD). To better understand the cellular and molecular genetic aspects of this disorder, we generated transgenic C. elegans strains expressing either the wild-type (ATM) or Z mutant form (ATZ) of the human serpin fused to GFP. Animals secreted ATM, but retained polymerized ATZ within dilated ER cisternae. These latter animals also showed slow growth, smaller brood sizes and decreased longevity; phenotypes observed in ATD patients or transgenic mouse lines expressing ATZ. Similar to mammalian models, ATZ was disposed of by autophagy and ER-associated degradation pathways. Mutant strains defective in insulin signaling (daf-2) also showed a marked decrease in ATZ accumulation. Enhanced ATZ turnover was associated with the activity of two proteins central to systemic/exogenous (exo)-RNAi pathway: the dsRNA importer, SID-1 and the argonaute, RDE-1. Animals with enhanced exo-RNAi activity (rrf-3 mutant) phenocopied the insulin signaling mutants and also showed increased ATZ turnover. Taken together, these studies allude to the existence of a novel proteostasis pathway that mechanistically links misfolded protein turnover to components of the systemic RNAi machinery.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

