Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus
- PMID: 24838349
- PMCID: PMC4146708
- DOI: 10.1002/art.38703
Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus
Abstract
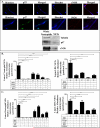
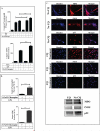
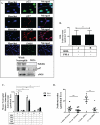
Objective: Oxidative stress and oxidized high-density lipoprotein (HDL) are implicated as risk factors for cardiovascular disease (CVD) in systemic lupus erythematosus (SLE). Yet, how HDL is oxidized and rendered dysfunctional in SLE remains unclear. Neutrophil extracellular traps (NETs), the levels of which are elevated in lupus, possess oxidant-generating enzymes, including myeloperoxidase (MPO), NADPH oxidase (NOX), and nitric oxide synthase (NOS). We hypothesized that NETs mediate HDL oxidation, impairing cholesterol efflux capacity (CEC).
Methods: Plasma MPO levels and CEC activity were examined in controls and lupus patients, and 3-chlorotyrosine (MPO specific) and 3-nitrotyrosine (derived from reactive nitrogen species) were quantified in human HDL. Multivariable linear models were used to estimate and test differences between groups. HDL was exposed to NETs from control and lupus neutrophils in the presence or absence of MPO, NOX, NOS inhibitors, and chloroquine (CQ). Murine HDL oxidation was quantified after NET inhibition in vivo.
Results: SLE patients displayed higher MPO levels and diminished CEC compared to controls. SLE HDL had higher 3-nitrotyrosine and 3-chlorotyrosine content than control HDL, with site-specific oxidation signatures on apolipoprotein A-I. Experiments with human and murine NETs confirmed that chlorination was mediated by MPO and NOX, and nitration by NOS and NOX. Mice with lupus treated with the NET inhibitor Cl-amidine displayed significantly decreased HDL oxidation. CQ inhibited NET formation in vitro.
Conclusion: Active NOS, NOX, and MPO within NETs significantly modify HDL, rendering the lipoprotein proatherogenic. Since NET formation is enhanced in SLE, these findings support a novel role for NET-derived lipoprotein oxidation in SLE-associated CVD and identify additional proatherogenic roles of neutrophils and putative protective roles of antimalarials in autoimmunity.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. - PubMed
-
- Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73(3):609–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL112625/HL/NHLBI NIH HHS/United States
- DK-089503/DK/NIDDK NIH HHS/United States
- Z99 AR999999/ImNIH/Intramural NIH HHS/United States
- R01 HL088419/HL/NHLBI NIH HHS/United States
- R01 GM079357/GM/NIGMS NIH HHS/United States
- GM-079357/GM/NIGMS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- U24 DK097153/DK/NIDDK NIH HHS/United States
- T32 AI007413/AI/NIAID NIH HHS/United States
- DK-097153/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- AI-007413/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

