TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury
- PMID: 24838754
- PMCID: PMC4152165
- DOI: 10.1152/ajplung.00065.2014
TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury
Abstract
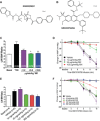
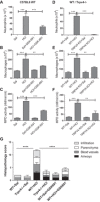
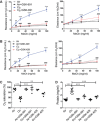
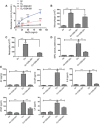
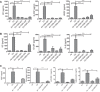
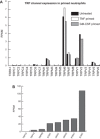
The treatment of acute lung injury caused by exposure to reactive chemicals remains challenging because of the lack of mechanism-based therapeutic approaches. Recent studies have shown that transient receptor potential vanilloid 4 (TRPV4), an ion channel expressed in pulmonary tissues, is a crucial mediator of pressure-induced damage associated with ventilator-induced lung injury, heart failure, and infarction. Here, we examined the effects of two novel TRPV4 inhibitors in mice exposed to hydrochloric acid, mimicking acid exposure and acid aspiration injury, and to chlorine gas, a severe chemical threat with frequent exposures in domestic and occupational environments and in transportation accidents. Postexposure treatment with a TRPV4 inhibitor suppressed acid-induced pulmonary inflammation by diminishing neutrophils, macrophages, and associated chemokines and cytokines, while improving tissue pathology. These effects were recapitulated in TRPV4-deficient mice. TRPV4 inhibitors had similar anti-inflammatory effects in chlorine-exposed mice and inhibited vascular leakage, airway hyperreactivity, and increase in elastance, while improving blood oxygen saturation. In both models of lung injury we detected increased concentrations of N-acylamides, a class of endogenous TRP channel agonists. Taken together, we demonstrate that TRPV4 inhibitors are potent and efficacious countermeasures against severe chemical exposures, acting against exaggerated inflammatory responses, and protecting tissue barriers and cardiovascular function.
Keywords: TRPV4; acute lung injury; chlorine.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- Becker M, Forrester M. Pattern of chlorine gas exposures reported to Texas poison control centers, 2000 through 2005. Tex Med 104: 52–57, 51, 2008 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

