Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis
- PMID: 24839206
- PMCID: PMC4314719
- DOI: 10.1002/art.38699
Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis
Abstract
Objective: To assess the efficacy and safety of rilonacept, an interleukin-1 inhibitor, in a randomized, double-blind, placebo-controlled trial.
Methods: An initial 4-week double-blind placebo phase was incorporated into a 24-week randomized multicenter design, followed by an open-label phase. Seventy-one children who had active arthritis in ≥2 joints were randomized (1:1) to the 2 arms of the study. Patients in the rilonacept arm received rilonacept (loading dose 4.4 mg/kg followed by 2.2 mg/kg weekly, subcutaneously) beginning on day 0. Patients in the placebo arm received placebo for 4 weeks followed by a loading dose of rilonacept at week 4 followed by weekly maintenance doses. The primary end point was time to response, using the adapted American College of Rheumatology Pediatric 30 criteria coupled with the absence of fever and taper of the dosage of systemic corticosteroids, using prespecified criteria.
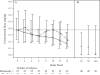
Results: The time to response was shorter in the rilonacept arm than in the placebo arm (χ(2) = 7.235, P = 0.007). The secondary analysis, which used the same response criteria, showed that 20 (57%) of 35 patients in the rilonacept arm had a response at week 4 compared with 9 (27%) of 33 patients in the placebo arm (P = 0.016). Exacerbation of systemic juvenile idiopathic arthritis (JIA) was the most common severe adverse event. More patients in the rilonacept arm had elevated liver transaminase levels (including levels more than 3 times the upper limit of normal) compared with those in the placebo arm. Adverse events were similar in the 2 arms of the study.
Conclusion: Rilonacept was generally well tolerated and demonstrated efficacy in active systemic JIA.
Copyright © 2014 by the American College of Rheumatology.
Figures


Comment in
-
Reply: To PMID 24839206.Arthritis Rheumatol. 2015 Mar;67(3):858. doi: 10.1002/art.38991. Arthritis Rheumatol. 2015. PMID: 25511916 No abstract available.
-
Half-life and safety of canakinumab in pediatric patients: comment on the article by Ilowite et Al.Arthritis Rheumatol. 2015 Mar;67(3):857-8. doi: 10.1002/art.38992. Arthritis Rheumatol. 2015. PMID: 25512026 Free PMC article. No abstract available.
References
-
- Grom AA, Passo M. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis. J Pediatr. 1996;129:630–2. - PubMed
-
- Singh-Grewal D, Schneider R, Bayer N, Feldman BM. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 2006;54:1595–601. - PubMed
-
- Lomater C, Gerloni V, Gattinara M, Mazzotti J, Cimaz R, Fantini F. Systemic onset juvenile idiopathic arthritis: a retrospective study of 80 consecutive patients followed for 10 years. J Rheumatol. 2000;27:491–6. - PubMed
-
- Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol. 2006;2:28–34. - PubMed
-
- Spiegel LR, Schneider R, Lang BA, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- TR000006/TR/NCATS NIH HHS/United States
- HHSN268200700015C/AR/NIAMS NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- 1 UL1-RR025014-01/RR/NCRR NIH HHS/United States
- 3 UL1 TR 000086-05S1/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- UL1 RR025750/RR/NCRR NIH HHS/United States
- N01-AR-700015/AR/NIAMS NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

