No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory
- PMID: 24839903
- PMCID: PMC4042179
- DOI: 10.1038/cr.2014.65
No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory
Abstract
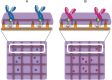
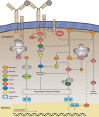
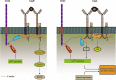
Acquired immunological memory is a striking phenomenon. A lethal epidemic sweeps through a naïve population, many die but those who survive are never "attacked twice - never at least fatally", as the historian Thucydides observed in 430 BCE. Antibody memory is critical for protection against many human infectious diseases and is the basis for nearly all current human vaccines. Antibody memory is encoded, in part, in isotype-switched immunoglobulin (Ig)G-expressing memory B cells that are generated in the primary response to antigen and give rise to rapid, high-affinity and high-titered antibody responses upon challenge with the same antigen. How IgG-B-cell receptors (BCRs) and antigen-induced IgG-BCR signaling contribute to memory antibody responses are not fully understood. In this review, we summarize exciting new advances that are revealing the cellular and molecular mechanisms at play in antibody memory and discuss how studies using different experimental approaches will help elucidate the complex phenomenon of B-cell memory.
Figures

Similar articles
-
The immunoglobulin tail tyrosine motif upgrades memory-type BCRs by incorporating a Grb2-Btk signalling module.Nat Commun. 2014 Nov 21;5:5456. doi: 10.1038/ncomms6456. Nat Commun. 2014. PMID: 25413232 Free PMC article.
-
How B cells remember? A sophisticated cytoplasmic tail of mIgG is pivotal for the enhanced transmembrane signaling of IgG-switched memory B cells.Prog Biophys Mol Biol. 2015 Sep;118(3):89-94. doi: 10.1016/j.pbiomolbio.2015.04.010. Epub 2015 May 21. Prog Biophys Mol Biol. 2015. PMID: 26004919 Review.
-
Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells.Nat Immunol. 2009 Sep;10(9):1018-25. doi: 10.1038/ni.1764. Epub 2009 Aug 9. Nat Immunol. 2009. PMID: 19668218
-
Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors.J Exp Med. 2007 Apr 16;204(4):759-69. doi: 10.1084/jem.20061923. Epub 2007 Apr 9. J Exp Med. 2007. PMID: 17420266 Free PMC article.
-
Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses.Cancer Immunol Res. 2014 May;2(5):381-92. doi: 10.1158/2326-6066.CIR-14-0029. Cancer Immunol Res. 2014. PMID: 24795350 Review.
Cited by
-
Epigenetic Regulation of Antibody Responses by the Histone H2A Deubiquitinase MYSM1.Sci Rep. 2015 Sep 8;5:13755. doi: 10.1038/srep13755. Sci Rep. 2015. PMID: 26348977 Free PMC article.
-
Naïve and memory B cells exhibit distinct biochemical responses following BCR engagement.Immunol Cell Biol. 2016 Sep;94(8):774-86. doi: 10.1038/icb.2016.41. Epub 2016 Apr 22. Immunol Cell Biol. 2016. PMID: 27101923
-
Antigen delivery format variation and formulation stability through use of a hybrid vector.Vaccine X. 2019 Jan 30;1:100012. doi: 10.1016/j.jvacx.2019.100012. eCollection 2019 Apr 11. Vaccine X. 2019. PMID: 31384734 Free PMC article.
-
Germinal Center B Cell Dynamics.Immunity. 2016 Sep 20;45(3):471-482. doi: 10.1016/j.immuni.2016.09.001. Immunity. 2016. PMID: 27653600 Free PMC article. Review.
-
Ion channel TRPV2 is critical in enhancing B cell activation and function.J Exp Med. 2024 Mar 4;221(3):e20221042. doi: 10.1084/jem.20221042. Epub 2024 Feb 14. J Exp Med. 2024. PMID: 38353705 Free PMC article.
References
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. - PubMed
-
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. - PubMed
-
- Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. - PubMed
-
- Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

