Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information
- PMID: 24839936
- PMCID: PMC5528579
- DOI: 10.1016/j.molonc.2014.04.006
Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information
Abstract
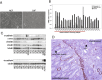
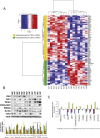
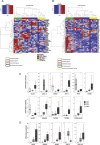
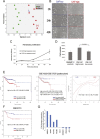
Little is known about the difference in gene expression between carcinoma-associated fibroblasts (CAFs) and paired normal colonic fibroblasts (NCFs) in colorectal cancer. Paired CAFs and NCFs were isolated from eight primary human colorectal carcinoma specimens. In culture conditions, soluble factors secreted by CAFs in the conditioned media increased clonogenicity and migration of epithelial cancer cells lines to a greater extent than did NCF. In vivo, CAFs were more competent as tumour growth enhancers than paired NCFs when co-inoculated with colorectal cell lines. Gene expression analysis of microarrays of CAF and paired NCF populations enabled us to identify 108 deregulated genes (38 upregulated and 70 downregulated genes). Most of those genes are fibroblast-specific. This has been validated in silico in dataset GSE39396 and by qPCR in selected genes. GSEA analysis revealed a differential transcriptomic profile of CAFs, mainly involving the Wnt signallingsignalling pathway, focal adhesion and cell cycle. Both deregulated genes and biological processes involved depicted a considerable degree of overlap with deregulated genes reported in breast, lung, oesophagus and prostate CAFs. These observations suggest that similar transcriptomic programs may be active in the transition from normal fibroblast in adjacent tissues to CAFs, independently of their anatomic demarcation. Additionally NCF already depicted an activated pattern associated with inflammation. The deregulated genes signature score seemed to correlate with CAF tumour promoter abilities in vitro, suggesting a high degree of heterogeneity between CAFs, and it has also prognostic value in two independent datasets. Further characterization of the roles these biomarkers play in cancer will reveal how CAFs provide cancer cells with a suitable microenvironment and may help in the development of new therapeutic targets for cancer treatment.
Keywords: Biomarker; Carcinoma-associated fibroblasts; Colorectal; Microenvironment; Stroma.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Berdiel-Acer, M. , Bohem, M.E. , Lopez-Doriga, A. , Vidal, A. , Salazar, R. , Martinez-Iniesta, M. , Santos, C. , Sanjuan, X. , Villanueva, A. , Mollevi, D.G. , 2011. Hepatic carcinoma-associated fibroblasts promote an adaptative response in colorectal cancer cells that inhibit proliferation and apoptosis: nonresistant cells die by nonapoptotic cell death. Neoplasia. 13, 931–946. - PMC - PubMed
-
- Bissell, M.J. , Hall, H.G. , Parry, G. , 1982. How does the extracellular matrix direct gene expression?. J. Theor. Biol. 99, 31–68. - PubMed
-
- Calon, A. , Espinet, E. , Palomo-Ponce, S. , Tauriello, D.V. , Iglesias, M. , Cespedes, M.V. , Sevillano, M. , Nadal, C. , Jung, P. , Zhang, X.H. , Byrom, D. , Riera, A. , Rossell, D. , Mangues, R. , Massague, J. , Sancho, E. , Batlle, E. , 2012. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 22, 571–584. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

