CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity
- PMID: 24839957
- PMCID: PMC4159569
- DOI: 10.1007/s00401-014-1291-1
CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity
Abstract
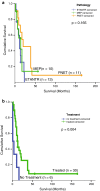
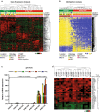
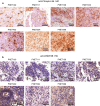
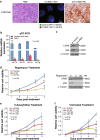
Amplification of the C19MC oncogenic miRNA cluster and high LIN28 expression has been linked to a distinctly aggressive group of cerebral CNS-PNETs (group 1 CNS-PNETs) arising in young children. In this study, we sought to evaluate the diagnostic specificity of C19MC and LIN28, and the clinical and biological spectra of C19MC amplified and/or LIN28+ CNS-PNETs. We interrogated 450 pediatric brain tumors using FISH and IHC analyses and demonstrate that C19MC alteration is restricted to a sub-group of CNS-PNETs with high LIN28 expression; however, LIN28 immunopositivity was not exclusive to CNS-PNETs but was also detected in a proportion of other malignant pediatric brain tumors including rhabdoid brain tumors and malignant gliomas. C19MC amplified/LIN28+ group 1 CNS-PNETs arose predominantly in children <4 years old; a majority arose in the cerebrum but 24 % (13/54) of tumors had extra-cerebral origins. Notably, group 1 CNS-PNETs encompassed several histologic classes including embryonal tumor with abundant neuropil and true rosettes (ETANTR), medulloepithelioma, ependymoblastoma and CNS-PNETs with variable differentiation. Strikingly, gene expression and methylation profiling analyses revealed a common molecular signature enriched for primitive neural features, high LIN28/LIN28B and DNMT3B expression for all group 1 CNS-PNETs regardless of location or tumor histology. Our collective findings suggest that current known histologic categories of CNS-PNETs which include ETANTRs, medulloepitheliomas, ependymoblastomas in various CNS locations, comprise a common molecular and diagnostic entity and identify inhibitors of the LIN28/let7/PI3K/mTOR axis and DNMT3B as promising therapeutics for this distinct histogenetic entity.
Figures

Comment in
-
Embryonal tumor with multilayered rosettes (ETMR): signed, sealed, delivered ….Acta Neuropathol. 2014 Aug;128(2):305-8. doi: 10.1007/s00401-014-1320-0. Acta Neuropathol. 2014. PMID: 25012402 No abstract available.
References
-
- Buccoliero AM, Castiglione F, Rossi Degl’Innocenti D, et al. Embryonal tumor with abundant neuropil and true rosettes: morphological, immunohistochemical, ultrastructural and molecular study of a case showing features of medulloepithelioma and areas of mesenchymal and epithelial differentiation. Neuropathology. 2010;30:84–91. doi: 10.1111/j.1440-1789.2009.01040.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

