PHEX mimetic (SPR4-peptide) corrects and improves HYP and wild type mice energy-metabolism
- PMID: 24839967
- PMCID: PMC4026222
- DOI: 10.1371/journal.pone.0097326
PHEX mimetic (SPR4-peptide) corrects and improves HYP and wild type mice energy-metabolism
Erratum in
- PLoS One. 2014;9(6):e101192
Abstract
Context: PHEX or DMP1 mutations cause hypophosphatemic-rickets and altered energy metabolism. PHEX binds to DMP1-ASARM-motif to form a complex with α5β3 integrin that suppresses FGF23 expression. ASARM-peptides increase FGF23 by disrupting the PHEX-DMP1-Integrin complex. We used a 4.2 kDa peptide (SPR4) that binds to ASARM-peptide/motif to study the DMP1-PHEX interaction and to assess SPR4 for the treatment of energy metabolism defects in HYP and potentially other bone-mineral disorders.
Design: Subcutaneously transplanted osmotic pumps were used to infuse SPR4-peptide or vehicle (VE) into wild-type mice (WT) and HYP-mice (PHEX mutation) for 4 weeks.
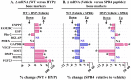
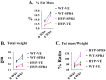
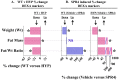
Results: SPR4 partially corrected HYP mice hypophosphatemia and increased serum 1.25(OH)2D3. Serum FGF23 remained high and PTH was unaffected. WT-SPR4 mice developed hypophosphatemia and hypercalcemia with increased PTH, FGF23 and 1.25(OH)2D3. SPR4 increased GAPDH HYP-bone expression 60× and corrected HYP-mice hyperglycemia and hypoinsulinemia. HYP-VE serum uric-acid (UA) levels were reduced and SPR4 infusion suppressed UA levels in WT-mice but not HYP-mice. SPR4 altered leptin, adiponectin, and sympathetic-tone and increased the fat mass/weight ratio for HYP and WT mice. Expression of perlipin-2 a gene involved in obesity was reduced in HYP-VE and WT-SPR4 mice but increased in HYP-SPR4 mice. Also, increased expression of two genes that inhibit insulin-signaling, ENPP1 and ESP, occurred with HYP-VE mice. In contrast, SPR4 reduced expression of both ENPP1 and ESP in WT mice and suppressed ENPP1 in HYP mice. Increased expression of FAM20C and sclerostin occurred with HYP-VE mice. SPR4 suppressed expression of FAM20C and sclerostin in HYP and WT mice.
Conclusions: ASARM peptides and motifs are physiological substrates for PHEX and modulate osteocyte PHEX-DMP1-α5β3-integrin interactions and thereby FGF23 expression. These interactions also provide a nexus that regulates bone and energy metabolism. SPR4 suppression of sclerostin and/or sequestration of ASARM-peptides improves energy metabolism and may have utility for treating familial rickets, osteoporosis, obesity and diabetes.
Conflict of interest statement
Figures




References
-
- Yamaguchi T, Sugimoto T (2011) Bone metabolism and fracture risk in type 2 diabetes mellitus [Review]. Endocrine journal 58: 613–624. - PubMed
-
- Jemtland R, Holden M, Reppe S, Olstad OK, Reinholt FP, et al. (2011) Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. Journal of bone and mineral research 26: 1793–1801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

