Streocontrolled construction of six vicinal stereogenic centers on spiropyrazolones via organocascade Michael/Michael/1,2-addition reactions
- PMID: 24840166
- PMCID: PMC4699267
- DOI: 10.1021/ol501093v
Streocontrolled construction of six vicinal stereogenic centers on spiropyrazolones via organocascade Michael/Michael/1,2-addition reactions
Abstract
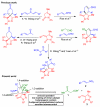
A highly stereoselective one-pot procedure for the synthesis of spiropyrazolone derivatives bearing six contiguous stereogenic centers including two tetrasubstituted carbons has been developed. Under sequential catalysis by two organocatalysts, a cinchona-derived aminosquaramide and DBU, a series of diversely functionalized spiropyrazolones are obtained in good yields (47-62%) and excellent stereoselectivities (up to >25:1 dr and 98-99% ee). The opposite enantiomers of the spiropyrazolones are also accessible by employing a pseudoenantiomeric aminosquaramide catalyst.
Figures


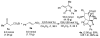
Similar articles
-
Highly diastereoselective synthesis of spiropyrazolones.Molecules. 2015 May 13;20(5):8574-82. doi: 10.3390/molecules20058574. Molecules. 2015. PMID: 25985358 Free PMC article.
-
Core scaffold-inspired stereoselective synthesis of spiropyrazolones via an organocatalytic Michael/cyclization sequence.J Org Chem. 2012 Nov 16;77(22):10228-34. doi: 10.1021/jo301851a. Epub 2012 Oct 30. J Org Chem. 2012. PMID: 23072426
-
An organocatalytic asymmetric double Michael cascade reaction of unsaturated ketones and unsaturated pyrazolones: highly efficient synthesis of spiropyrazolone derivatives.Org Biomol Chem. 2013 Mar 7;11(9):1441-5. doi: 10.1039/c2ob27095a. Org Biomol Chem. 2013. PMID: 23262465
-
Recyclable fluorous cinchona organocatalysts for asymmetric synthesis of biologically interesting compounds.Chem Commun (Camb). 2021 Oct 5;57(79):10116-10124. doi: 10.1039/d1cc03722f. Chem Commun (Camb). 2021. PMID: 34522921 Review.
-
Non-Covalent Organocatalyzed Domino Reactions Involving Oxindoles: Recent Advances.Molecules. 2017 Sep 29;22(10):1636. doi: 10.3390/molecules22101636. Molecules. 2017. PMID: 28961217 Free PMC article. Review.
Cited by
-
Highly diastereoselective synthesis of spiropyrazolones.Molecules. 2015 May 13;20(5):8574-82. doi: 10.3390/molecules20058574. Molecules. 2015. PMID: 25985358 Free PMC article.
-
Combining silver catalysis and organocatalysis: a sequential Michael addition/hydroalkoxylation one-pot approach to annulated coumarins.Org Lett. 2014 Oct 3;16(19):5188-91. doi: 10.1021/ol502551u. Epub 2014 Sep 24. Org Lett. 2014. PMID: 25250728 Free PMC article.
-
Organocatalytic Asymmetric Synthesis of Dihydroisoquinolinones via a One-Pot Aza-Henry-Hemiaminalization-Oxidation Sequence.Synthesis (Stuttg). 2015 Feb 1;47(4):472-480. doi: 10.1055/s-0034-1379398. Synthesis (Stuttg). 2015. PMID: 26722132 Free PMC article.
-
Asymmetric Synthesis of Spiropyrazolones by Sequential Organo- and Silver Catalysis.Angew Chem Int Ed Engl. 2016 Jan 26;55(5):1797-800. doi: 10.1002/anie.201510602. Epub 2015 Dec 16. Angew Chem Int Ed Engl. 2016. PMID: 26676875 Free PMC article.
-
Combined inorganic base promoted N-addition/[2,3]-sigmatropic rearrangement to construct homoallyl sulfur-containing pyrazolones.RSC Adv. 2019 Oct 29;9(60):34912-34925. doi: 10.1039/c9ra07610g. eCollection 2019 Oct 28. RSC Adv. 2019. PMID: 35542052 Free PMC article.
References
-
-
For a book on pyrazolone chemistry, see:
- Varvounis G. Pyrazol-3-ones. Part IV: Synthesis and Applications. In: Katritzky AR, editor. Advances in Heterocyclic Chemistry. Vol. 98. Academic Press; New York: 2009. p. 143.
-
For reviews, see:
- Horton DA, Bourne GT, Smythe ML. Chem. Rev. 2003;103:893. - PubMed
- Watanabe T, Tanaka K, Watanabe K, Takamatsu Y, Tobe A. Yakugaku Zasshi. 2004;124:99. - PubMed
- Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. CNS Drug Rev. 2006;12:9. - PMC - PubMed
-
For selected examples, see:
- Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF, Brune K. FASEB J. 2007;21:2343. - PubMed
- Wong A, Sibbald A, Ferrero F, Plager M, Santolaya ME, Escobar AM, Campos S, Barragan S, Gonzalez MDL, Kesselring GLF. Clin. Pediatr. 2001;40:313. - PubMed
- Hadi V, Koh Y-H, Sanchez TW, Barrios D, Neamati N, Jung KW. Bioorg. Med. Chem. Lett. 2010;20:6854. - PMC - PubMed
- Uvebrant K, Thrige DDG, Rosén A, Åkesson M, Berg H, Walse B, Björk P. J. Biomol. Screen. 2007;12:464. - PubMed
- Kimata A, Nakagawa H, Ohyama R, Fukuuchi T, Ohta S, Suzuki T, Miyata N. J. Med. Chem. 2007;50:5053. - PubMed
- Golebiowski A, Townes JA, Laufersweiler MJ, Brugel TA, Clark MP, Clark CM, Jane FD, Laughlin SK, Sabat MP, Bookland RG, VanRens JC, De B, Hsieh LC, Janusz MJ, Walter RL, Webster ME, Mekel MJ. Bioorg. Med. Chem. Lett. 2005;15:2285. - PubMed
- Clark MP, Laughlin SK, Laufersweiler MJ, Bookland RG, Brugel TA, Golebiowski A, Sabat MP, Townes JA, VanRens JC, Djung JF, Natchus MG, De B, Hsieh LC, Xu SC, Walter RL, Mekel MJ, Heitmeyer SA, Brown KK, Juergens K, Taiwo YO, Janusz MJ. J. Med. Chem. 2004;47:2724. - PubMed
- Wang XH, Wang XK, Liang YJ, Shi Z, Zhang JY, Chen LM, Fu LW. Chin. J. Cancer. 2010;29:980. - PubMed
-
-
-
For recent examples of the construction of pyrazolone derivatives, see:
- Gogoi S, Zhao C-G. Tetrahedron Lett. 2009;50:2252. - PMC - PubMed
- Gogoi S, Zhao C-G, Ding D. Org. Lett. 2009;11:2249. - PMC - PubMed
- Mariappan G, Saha BP, Bhuyan NR, Bharti PR, Kumar DJ. Adv. Pharm. Technol. Res. 2010;1:260. - PMC - PubMed
- Ma R, Zhu J, Liu J, Chen L, Shen X, Jiang H, Li J. Molecules. 2010;15:3593. - PMC - PubMed
- Liao Y-H, Chen W-B, Wu Z-J, Du X-L, Cun L-F, Zhang X-M, Yuan W-C. Adv. Synth. Catal. 2010;352:827.
- Yang Z, Wang Z, Bai S, Liu X, Lin L, Feng X. Org. Lett. 2011;13:596. - PubMed
- Mazzanti A, Calbet T, Font-Bardia M, Moyano A, Rios R. Org. Biomol. Chem. 2012;10:1645. - PubMed
- Šimek M, Remeš M, Veselý J, Rios R. Asian J. Org. Chem. 2013;2:64.
-
-
-
For recent reviews on the stereoselective synthesis of spirocyclic compounds, see:
- Trost BM, Brennan MK. Synthesis. 2009:3003.
- Rios R. Chem. Soc. Rev. 2012;41:1060. - PubMed
- Nakazakia A, Kobayashi S. Synlett. 2012:1427.
- Franz AK, Hanhan NV, Ball-Jones NR. ACS Catal. 2013;3:540.
-
-
-
For selected reviews on organocatalytic domino/cascade reactions, see:
- Enders D, Grondal C, Hűttl MRM. Angew. Chem., Int. Ed. 2007;46:1570. - PubMed
- Yu X, Wang W. Org. Biomol. Chem. 2008;6:2037. - PubMed
- Grondal C, Jeanty M, Enders D. Nat. Chem. 2010;2:167. - PubMed
- Albrecht Ł, Jiang H, Jørgensen KA. Angew. Chem., Int. Ed. 2011;50:8492. - PubMed
- Moyano A, Rios R. Chem. Rev. 2011;111:4703. - PubMed
- Grossmann A, Enders D. Angew. Chem., Int. Ed. 2012;51:314. - PubMed
- Pellissier H. Adv. Synth. Catal. 2012;354:237.
- Loh CCJ, Enders D. Chem.—Eur. J. 2012;18:10212. - PubMed
- Lu L-Q, Chen J-R, Xiao W-J. Acc. Chem. Res. 2012;45:1278. - PubMed
- Volla CMR, Atodiresei I, Rueping M. Chem. Rev. 2014;114:2390. - PubMed
-
For recent highlights, see:
- Chauhan P, Enders D. Angew. Chem., Int. Ed. 2014;53:1485. - PubMed
-
-
-
For recent reviews on the organocatalytic asymmetric synthesis of spirooxindoles, see:
- Zhou F, Liu Y-L, Zhou J. Adv. Synth. Catal. 2010;352:1381.
- Badillo JJ, Hanhan NV, Franz AK. Curr. Opin. Drug Discovery Dev. 2010;13:758. - PubMed
- Dalpozzo R, Bartoli G, Bencivenni G. Chem. Soc. Rev. 2012;41:7247. - PubMed
- Ball-Jones NR, Badillo JJ, Franz AK. Org. Biomol. Chem. 2012;10:5165. - PubMed
- Hong L, Wang R. Adv. Synth. Catal. 2013;355:1023.
- Liu Y, Wang H, Wan J. Asian J. Org. Chem. 2013;2:374. - PMC - PubMed
- Chauhan P, Chimni SS. Tetrahedron: Asymmetry. 2013;24:343.
- Cheng D, Ishihara Y, Tan B, Barbas CF., III ACS Catal. 2014;4:743.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

