The microbiota, the immune system and the allograft
- PMID: 24840316
- PMCID: PMC4423796
- DOI: 10.1111/ajt.12760
The microbiota, the immune system and the allograft
Abstract
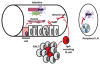
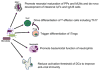
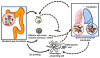
The microbiota represents the complex collections of microbial communities that colonize a host. In health, the microbiota is essential for metabolism, protection against pathogens and maturation of the immune system. In return, the immune system determines the composition of the microbiota. Altered microbial composition (dysbiosis) has been correlated with a number of diseases in humans. The tight reciprocal immune/microbial interactions complicate determining whether dysbiosis is a cause and/or a consequence of immune dysregulation and disease initiation or progression. However, a number of studies in germ-free and antibiotic-treated animal models support causal roles for intestinal bacteria in disease susceptibility. The role of the microbiota in transplant recipients is only starting to be investigated and its study is further complicated by putative contributions of both recipient and donor microbiota. Moreover, both flora may be affected directly or indirectly by immunosuppressive drugs and antimicrobial prophylaxis taken by transplant patients, as well as by inflammatory processes secondary to ischemia/reperfusion and allorecognition, and the underlying cause of end-organ failure. Whether the ensuing dysbiosis affects alloresponses and whether therapies aimed at correcting dysbiosis should be considered in transplant patients constitutes an exciting new field of research.
Keywords: Acute rejection; alloimmunity; chronic rejection; microbiota; pattern-recognition receptor.
© Copyright 2014 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
Similar articles
-
The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088
-
Microbiome analysis, the immune response and transplantation in the era of next generation sequencing.Hum Immunol. 2021 Nov;82(11):883-901. doi: 10.1016/j.humimm.2021.07.009. Epub 2021 Aug 5. Hum Immunol. 2021. PMID: 34364710
-
Drosophila as a model for intestinal dysbiosis and chronic inflammatory diseases.Dev Comp Immunol. 2014 Jan;42(1):102-10. doi: 10.1016/j.dci.2013.05.005. Epub 2013 May 16. Dev Comp Immunol. 2014. PMID: 23685204 Review.
-
The Interplay between Gut Microbiota and the Immune System in Liver Transplant Recipients and Its Role in Infections.Infect Immun. 2021 Oct 15;89(11):e0037621. doi: 10.1128/IAI.00376-21. Epub 2021 Aug 30. Infect Immun. 2021. PMID: 34460287 Free PMC article. Review.
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
Cited by
-
Impact of donor multidrug-resistant organisms on solid organ transplant recipient outcomes.Transpl Infect Dis. 2022 Feb;24(1):e13783. doi: 10.1111/tid.13783. Epub 2022 Jan 10. Transpl Infect Dis. 2022. PMID: 34968006 Free PMC article.
-
Diverse Routes of Allograft Tolerance Disruption by Memory T Cells.Front Immunol. 2020 Oct 8;11:580483. doi: 10.3389/fimmu.2020.580483. eCollection 2020. Front Immunol. 2020. PMID: 33117387 Free PMC article. Review.
-
Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease.Front Immunol. 2022 Jul 28;13:926550. doi: 10.3389/fimmu.2022.926550. eCollection 2022. Front Immunol. 2022. PMID: 35967386 Free PMC article. Review.
-
Impact of environmental factors on alloimmunity and transplant fate.J Clin Invest. 2017 Jun 30;127(7):2482-2491. doi: 10.1172/JCI90596. Epub 2017 May 8. J Clin Invest. 2017. PMID: 28481225 Free PMC article. Review.
-
Microbiome analysis reveals the inducing effect of Pseudomonas on prostatic hyperplasia via activating NF-κB signalling.Virulence. 2024 Dec;15(1):2313410. doi: 10.1080/21505594.2024.2313410. Epub 2024 Feb 20. Virulence. 2024. PMID: 38378443 Free PMC article.
References
-
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

