Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides
- PMID: 24840573
- PMCID: PMC4057757
- DOI: 10.3390/ijms15058753
Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides
Abstract
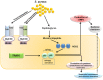
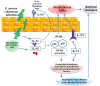
Staphylococcus aureus (S. aureus) is a widespread cutaneous pathogen responsible for the great majority of bacterial skin infections in humans. The incidence of skin infections by S. aureus reflects in part the competition between host cutaneous immune defenses and S. aureus virulence factors. As part of the innate immune system in the skin, cationic antimicrobial peptides (CAMPs) such as the β-defensins and cathelicidin contribute to host cutaneous defense, which prevents harmful microorganisms, like S. aureus, from crossing epithelial barriers. Conversely, S. aureus utilizes evasive mechanisms against host defenses to promote its colonization and infection of the skin. In this review, we focus on host-pathogen interactions during colonization and infection of the skin by S. aureus and methicillin-resistant Staphylococcus aureus (MRSA). We will discuss the peptides (defensins, cathelicidins, RNase7, dermcidin) and other mediators (toll-like receptor, IL-1 and IL-17) that comprise the host defense against S. aureus skin infection, as well as the various mechanisms by which S. aureus evades host defenses. It is anticipated that greater understanding of these mechanisms will enable development of more sustainable antimicrobial compounds and new therapeutic approaches to the treatment of S. aureus skin infection and colonization.
Figures

References
-
- Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. - PubMed
-
- Graham P.L., 3rd, Lin S. X., Larson E.L. A U.S. population-based survey of Staphylococcus aureus colonization. Ann. Intern. Med. 2006;144:318–325. - PubMed
-
- Kuehnert M.J., Kruszon-Moran D., Hill H.A., McQuillan G., McAllister S.K., Fosheim G., McDougal L.K., Chaitram J., Jensen B., Fridkin S.K., et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J. Infect. Dis. 2006;193:172–179. - PubMed
-
- Perl T.M., Cullen J.J., Wenzel R.P., Zimmerman M.B., Pfaller M.A., Sheppard D., Twombley J., French P.P., Herwaldt L.A., et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002;346:1871–1877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

