Structural basis for the nonlinear mechanics of fibrin networks under compression
- PMID: 24840618
- PMCID: PMC4104543
- DOI: 10.1016/j.biomaterials.2014.04.056
Structural basis for the nonlinear mechanics of fibrin networks under compression
Abstract
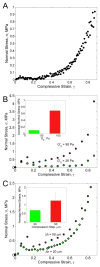
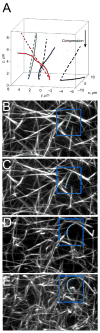
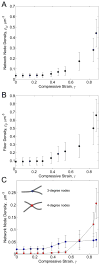
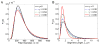
Fibrin is a protein polymer that forms a 3D filamentous network, a major structural component of protective physiological blood clots as well as life threatening pathological thrombi. It plays an important role in wound healing, tissue regeneration and is widely employed in surgery as a sealant and in tissue engineering as a scaffold. The goal of this study was to establish correlations between structural changes and mechanical responses of fibrin networks exposed to compressive loads. Rheological measurements revealed nonlinear changes of fibrin network viscoelastic properties under dynamic compression, resulting in network softening followed by its dramatic hardening. Repeated compression/decompression enhanced fibrin clot stiffening. Combining fibrin network rheology with simultaneous confocal microscopy provided direct evidence of structural modulations underlying nonlinear viscoelasticity of compressed fibrin networks. Fibrin clot softening in response to compression strongly correlated with fiber buckling and bending, while hardening was associated with fibrin network densification. Our results suggest a complex interplay of entropic and enthalpic mechanisms accompanying structural changes and accounting for the nonlinear mechanical response in fibrin networks undergoing compressive deformations. These findings provide new insight into the fibrin clot structural mechanics and can be useful for designing fibrin-based biomaterials with modulated viscoelastic properties.
Keywords: Compression; Confocal microscopy; Fibrin networks; Mechanical response; Network structure; Rheology.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening.J Thromb Haemost. 2017 May;15(5):938-949. doi: 10.1111/jth.13650. Epub 2017 Mar 6. J Thromb Haemost. 2017. PMID: 28166607
-
Contribution of nascent cohesive fiber-fiber interactions to the non-linear elasticity of fibrin networks under tensile load.Acta Biomater. 2019 Aug;94:514-523. doi: 10.1016/j.actbio.2019.05.068. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31152942 Free PMC article.
-
Compression-induced structural and mechanical changes of fibrin-collagen composites.Matrix Biol. 2017 Jul;60-61:141-156. doi: 10.1016/j.matbio.2016.10.007. Epub 2016 Oct 15. Matrix Biol. 2017. PMID: 27751946 Free PMC article.
-
Structure of fibrin: impact on clot stability.J Thromb Haemost. 2007 Jul;5 Suppl 1:116-24. doi: 10.1111/j.1538-7836.2007.02504.x. J Thromb Haemost. 2007. PMID: 17635717 Review.
Cited by
-
Recurrent venous thromboembolism patients form clots with lower elastic modulus than those formed by patients with non-recurrent disease.J Thromb Haemost. 2019 Apr;17(4):618-626. doi: 10.1111/jth.14402. Epub 2019 Mar 8. J Thromb Haemost. 2019. PMID: 30725502 Free PMC article.
-
What Is the Biological and Clinical Relevance of Fibrin?Semin Thromb Hemost. 2016 Jun;42(4):333-43. doi: 10.1055/s-0036-1571342. Epub 2016 Apr 7. Semin Thromb Hemost. 2016. PMID: 27056152 Free PMC article. Review.
-
A constitutive model for the time-dependent, nonlinear stress response of fibrin networks.Biomech Model Mechanobiol. 2015 Oct;14(5):995-1006. doi: 10.1007/s10237-015-0649-1. Epub 2015 Jan 25. Biomech Model Mechanobiol. 2015. PMID: 25618024 Free PMC article.
-
Acoustic and Elastic Properties of a Blood Clot during Microbubble-Enhanced Sonothrombolysis: Hardening of the Clot with Inertial Cavitation.Pharmaceutics. 2021 Sep 26;13(10):1566. doi: 10.3390/pharmaceutics13101566. Pharmaceutics. 2021. PMID: 34683859 Free PMC article.
-
Fibrinogen and Fibrin.Subcell Biochem. 2021;96:471-501. doi: 10.1007/978-3-030-58971-4_15. Subcell Biochem. 2021. PMID: 33252741 Review.
References
-
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–76. - PubMed
-
- Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–99. - PubMed
-
- Wolberg AS. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012;19:349–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

