Evaluation of oseltamivir prophylaxis regimens for reducing influenza virus infection, transmission and disease severity in a ferret model of household contact
- PMID: 24840623
- PMCID: PMC4130381
- DOI: 10.1093/jac/dku146
Evaluation of oseltamivir prophylaxis regimens for reducing influenza virus infection, transmission and disease severity in a ferret model of household contact
Abstract
Objectives: The emergence of the pandemic influenza A(H1N1)pdm09 virus in 2009 saw a significant increase in the therapeutic and prophylactic use of neuraminidase inhibitors (NAIs) to mitigate the impact of this highly transmissible virus. Prior to the pandemic, many countries stockpiled NAIs and developed pandemic plans for the use of antiviral drugs, based on either treatment of high-risk individuals and/or prophylaxis of contacts. However, to date there has been a lack of in vivo models to test the efficacy of treatment or prophylaxis with NAIs, for influenza-infected individuals or exposed contacts, in a household setting.
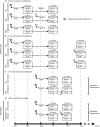
Methods: A ferret model of household contact was developed to study the efficacy of different prophylaxis regimens in preventing infection in contact ferrets exposed to influenza A(H1N1)pdm09-infected index ferrets.
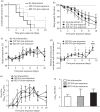
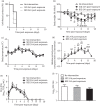
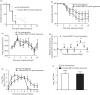
Results: Among the different prophylactic regimens, contact ferrets receiving oseltamivir prophylaxis twice daily showed better outcomes than those receiving oseltamivir once daily. Benefits included a significant delay in the time to secondary infection, lower weight loss and higher activity levels. The treatment of index ferrets at 36 h post-infection did not influence either secondary infection rates or clinical symptoms in exposed contact ferrets. Neither prophylaxis nor treatment prevented infection or reduced the duration of viral shedding, although clinical symptoms did improve in infected animals receiving prophylaxis.
Conclusions: Different oseltamivir prophylaxis regimens did not prevent infections, but consistently resulted in a reduction in symptoms in infected ferrets. However, oseltamivir prophylaxis failed to reduce viral titres, which warrants further investigation in humans.
Keywords: A(H1N1)pdm09; antiviral; neuraminidase inhibitors; pandemic.
© The Author 2014. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
Similar articles
-
Reduced airborne transmission of oseltamivir-resistant pandemic A/H1N1 virus in ferrets.Antivir Ther. 2011;16(5):775-9. doi: 10.3851/IMP1794. Antivir Ther. 2011. PMID: 21817200
-
Baloxavir treatment of ferrets infected with influenza A(H1N1)pdm09 virus reduces onward transmission.PLoS Pathog. 2020 Apr 15;16(4):e1008395. doi: 10.1371/journal.ppat.1008395. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32294137 Free PMC article.
-
Neuraminidase Mutations Conferring Resistance to Oseltamivir in Influenza A(H7N9) Viruses.J Virol. 2015 May;89(10):5419-26. doi: 10.1128/JVI.03513-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740997 Free PMC article.
-
Oseltamivir for treatment and prophylaxis of influenza infection.Expert Opin Drug Saf. 2009 May;8(3):357-71. doi: 10.1517/14740330902840519. Expert Opin Drug Saf. 2009. PMID: 19355841 Review.
-
[Role of neuraminidase inhibitors for the treatment of influenza A virus infections].Pathol Biol (Paris). 2010 Apr;58(2):e69-78. doi: 10.1016/j.patbio.2010.01.011. Epub 2010 Mar 19. Pathol Biol (Paris). 2010. PMID: 20303677 Review. French.
Cited by
-
Sequential Transmission of Influenza Viruses in Ferrets Does Not Enhance Infectivity and Does Not Predict Transmissibility in Humans.mBio. 2022 Dec 20;13(6):e0254022. doi: 10.1128/mbio.02540-22. Epub 2022 Oct 27. mBio. 2022. PMID: 36300929 Free PMC article.
-
Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models.Microbiol Mol Biol Rev. 2016 Jul 13;80(3):733-44. doi: 10.1128/MMBR.00022-16. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27412880 Free PMC article. Review.
-
Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model.Transl Res. 2020 Apr;218:16-28. doi: 10.1016/j.trsl.2019.12.002. Epub 2019 Dec 25. Transl Res. 2020. PMID: 31945316 Free PMC article.
-
The antiviral effects of baloxavir marboxil against influenza A virus infection in ferrets.Influenza Other Respir Viruses. 2020 Nov;14(6):710-719. doi: 10.1111/irv.12760. Epub 2020 Jun 13. Influenza Other Respir Viruses. 2020. PMID: 32533654 Free PMC article.
-
Oseltamivir inhibits influenza virus replication and transmission following ocular-only aerosol inoculation of ferrets.Virology. 2015 Oct;484:305-312. doi: 10.1016/j.virol.2015.06.020. Epub 2015 Jul 3. Virology. 2015. PMID: 26142497 Free PMC article.
References
-
- Bright RA, Medina MJ, Xu X, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–81. - PubMed
-
- Barr IG, Hurt AC, Deed N, et al. The emergence of adamantane resistance in influenza A(H1) viruses in Australia and regionally in 2006. Antiviral Res. 2007;75:173–6. - PubMed
-
- ChinaBioToday. 2013. China's SFDA Ready to Fast-Track Approvals of Peramivir, a Flu Treatmenthttp://www.chinabiotoday.com/articles/20130408_1. (13 March 2014, date last accessed)
-
- Chairat K, Tarning J, White NJ, et al. Pharmacokinetic properties of anti-influenza neuraminidase inhibitors. J Clin Pharmacol. 2013;53:119–39. - PubMed
-
- Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

